The treatment landscape for unresectable hepatocellular carcinoma (HCC) has shifted significantly with the integration of immune checkpoint inhibitors, including the STRIDE regimen combining tremelimumab and durvalumab. While its efficacy was established in the HIMALAYA trial, important uncertainties remain about how these therapies perform in routine clinical settings. The DT-Real study was developed to address this gap by examining real-world use of STRIDE and durvalumab monotherapy across an international, heterogeneous HCC population.
By capturing treatment patterns and clinical outcomes in both HIMALAYA-eligible and ineligible patients, DT-Real offers a clearer understanding of how immunotherapy is applied outside controlled trials and how underlying liver disease, tumor burden, and performance status shape the course of treatment in everyday practice.
Trial Design and Methods
DT-Real analyzed 233 patients with unresectable or metastatic HCC treated across 35 centers in Europe, the USA, and Asia. All received either STRIDE or durvalumab monotherapy between January 2022 and February 2025.
To mirror the HIMALAYA population, patients were divided into:
- HIMALAYA-IN: Child-Pugh A, ECOG 0–1, no prior systemic therapy, no Vp4 invasion
- HIMALAYA-OUT: those missing ≥1 criterion
About half the cohort (53%) met HIMALAYA eligibility, while the remainder represented a broader, more fragile real-world population.
The study assessed overall survival (OS), progression-free survival (PFS), objective response rate (ORR), and disease control rate (DCR) per RECIST v1.1. Hepatic decompensation—new ascites, encephalopathy, variceal bleeding, or jaundice—was evaluated using competing-risk methodology. Safety was characterized using CTCAE v5.0, and multivariable Cox and Fine–Gray models were used to identify predictors of outcomes. Sensitivity analyses were performed to ensure robustness.
Results
The findings of DT-Real, published in JHEP Reports, Articles in Press, November 20, 2025, show that STRIDE maintains meaningful clinical activity outside controlled trials. Median follow-up was six months.
In the full cohort, median OS was 20.4 months. Patients meeting HIMALAYA criteria performed markedly better, with a median OS of 23.0 months, compared with 12.2 months in the HIMALAYA-OUT group. In first-line therapy, Child-Pugh A patients reached 23.0 months, whereas Child-Pugh B patients had a shorter OS of 11.1 months. Macrovascular invasion and the development of hepatic decompensation were strong independent predictors of worse survival.
Median PFS was 6.0 months overall. HIMALAYA-IN patients achieved 6.6 months, and HIMALAYA-OUT patients 3.9 months. No significant differences in PFS were observed between STRIDE and durvalumab monotherapy, but these comparisons are limited by small subgroup sizes and selection bias.
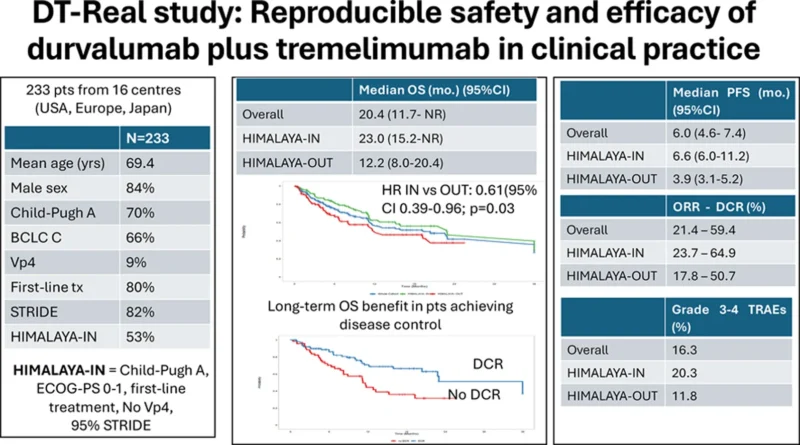
Among 187 evaluable patients, the objective response rate was 21.4%, with a disease control rate of 59.4%. Response outcomes favored HIMALAYA-IN patients, who had an ORR of 23.7%, compared with 17.8% in HIMALAYA-OUT patients. Achieving disease control (CR/PR/SD) was strongly associated with longer survival across subgroups.
New hepatic decompensation occurred in 13% of patients, most commonly as ascites. Incidence was similar between HIMALAYA-IN and HIMALAYA-OUT groups. Although baseline predictors were limited, hepatic decompensation itself was one of the strongest time-dependent drivers of mortality.
Safety
The median treatment duration was 6.2 months. Treatment-related adverse events occurred in 76.8% of patients, and 16.3% experienced grade 3–4 TRAEs. Overall, 5.1% discontinued therapy for toxicity, and 13.7% required high-dose corticosteroids for immune-related adverse events. The most frequent toxicities included skin reactions and diarrhoea/colitis, with diarrhoea/colitis and liver injury representing the most common grade 3–4 events. Safety patterns were similar across HIMALAYA eligibility groups and between STRIDE and monotherapy, although the small size of some subgroups limits strong comparative conclusions.
Conclusion
DT-Real provides important external validation for STRIDE in advanced HCC, demonstrating reproducible efficacy and tolerability in everyday clinical practice. Patients matching HIMALAYA criteria achieved numerically longer survival than in the pivotal trial, although this may reflect differences in baseline disease and limited follow-up rather than a true efficacy advantage.
However, nearly half of real-world patients fall outside those criteria—and their substantially poorer outcomes highlight ongoing unmet needs in HCC management. Careful patient selection, early monitoring for hepatic decompensation, and strategies tailored to those with impaired liver reserve will be essential.
As one of the largest real-world datasets for durvalumab-based therapy to date, the DT-Real study reinforces the central role of STRIDE in the treatment landscape while underscoring the importance of liver function and multidisciplinary decision-making in optimizing immunotherapy benefit.



