This multicenter retrospective study, conducted across 11 Swiss cancer centers, evaluated the role and timing of stereotactic radiotherapy (SRT) in patients with non-oncogene-addicted non-small cell lung cancer (NSCLC)presenting with asymptomatic brain metastases at diagnosis and treated with first-line immune checkpoint inhibitor (ICI)-based therapy.
Study Design and Population
A total of 128 patients were analyzed—58 received upfront SRT, while 69 were managed with systemic therapy alone. Eligible patients had 1–10 brain metastases (≤3 cm), ECOG PS 0–2, and no neurologic symptoms or corticosteroid use. Systemic regimens included ICI monotherapy, ICI + chemotherapy, or dual ICI combinations.
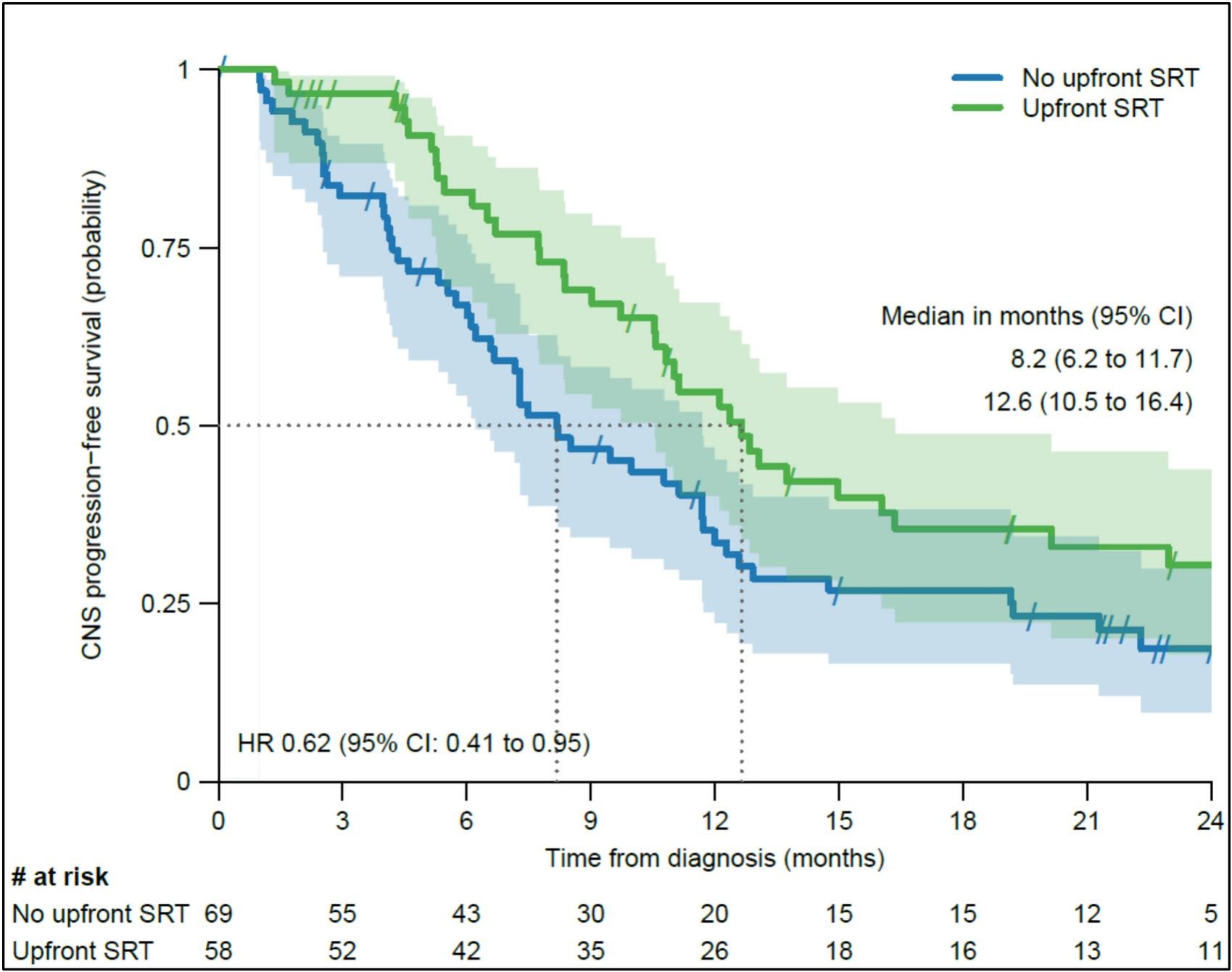
Efficacy Outcomes
After a median follow-up of 29 months, the median intracranial progression-free survival (PFS) was significantly longer in the SRT cohort compared to systemic therapy alone (12.6 vs. 8.2 months; HR 0.62, p = 0.026). This benefit persisted after adjustment for PD-L1 expression, lesion number, and size.
However, extracranial PFS (11.8 vs. 8.8 months, p = 0.26) and overall survival (22.8 vs. 21.7 months, p = 0.4) did not differ significantly between groups.
The intracranial objective response rate (ORR) was higher in the SRT group (71% vs. 54%; p = 0.049), although overall ORR showed no statistical difference (69% vs. 55%; p = 0.11). Notably, symptomatic brain progression was uncommon and comparable (3% vs. 12%; p = 0.11), indicating that close surveillance may safely defer local treatment in selected patients.
Safety
SRT was well tolerated with minimal CNS toxicity. Only two patients (3%) developed radiation necrosis, managed successfully with corticosteroids or anti-VEGF therapy. No fatal adverse events occurred.
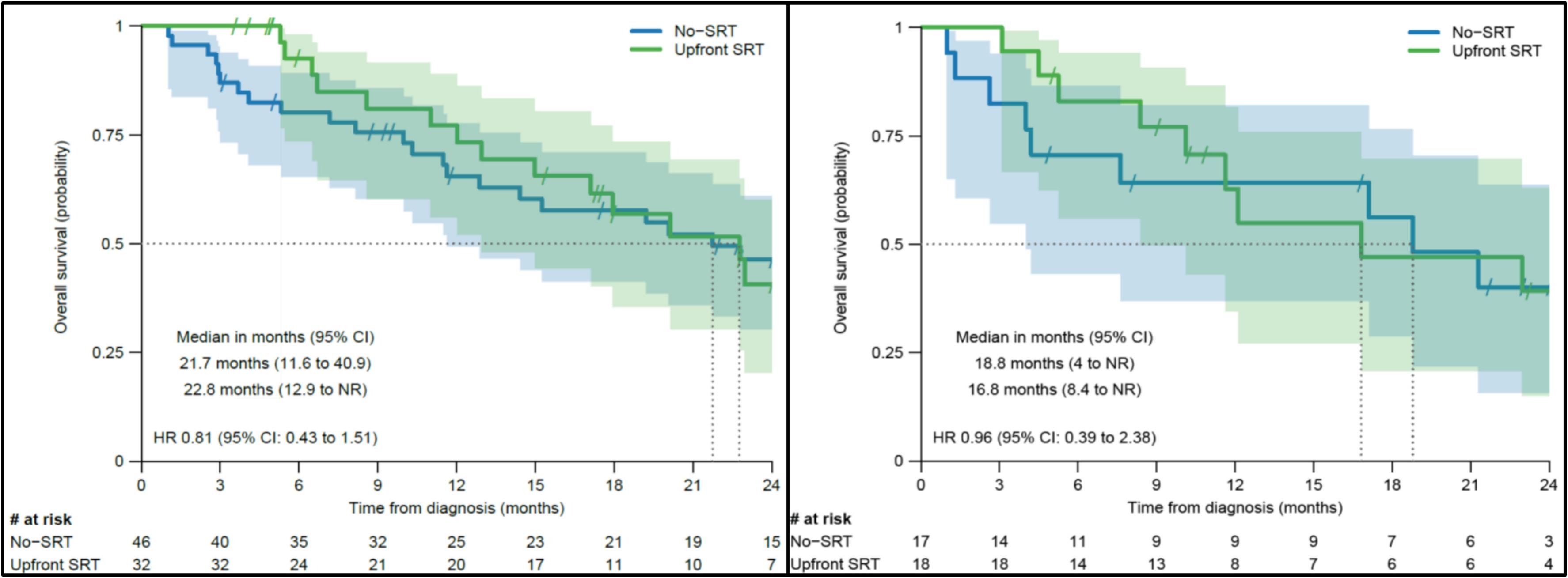
Interpretation
Upfront SRT significantly improved intracranial disease control without increasing toxicity but did not extend overall survival. Many patients in the non-SRT group never required local CNS therapy, suggesting that deferral of SRT may be appropriate for carefully monitored, asymptomatic patients receiving ICI-based systemic therapy with known CNS activity.
Clinical Implications
These findings support a personalized, surveillance-driven approach to brain metastases management in NSCLC, balancing intracranial control against treatment burden and toxicity. Prospective randomized trials such as ETOP STRIKE (NCT05522660) are warranted to define the optimal timing and patient selection for SRT in the era of effective systemic immunotherapy.
You Can Read All Article Here


