Immune checkpoint inhibitors (ICIs), either alone (IO) or combined with chemotherapy (chemo-IO), are standard for advanced NSCLC. However, “smoking history” is usually captured too crudely (ever/never), and it remains unclear how detailed tobacco exposure (status + pack-years) shapes clinical benefit and the underlying biology—especially in PD-L1–high disease where IO alone vs chemo-IO is a common first-line dilemma.
Methods and Study Design
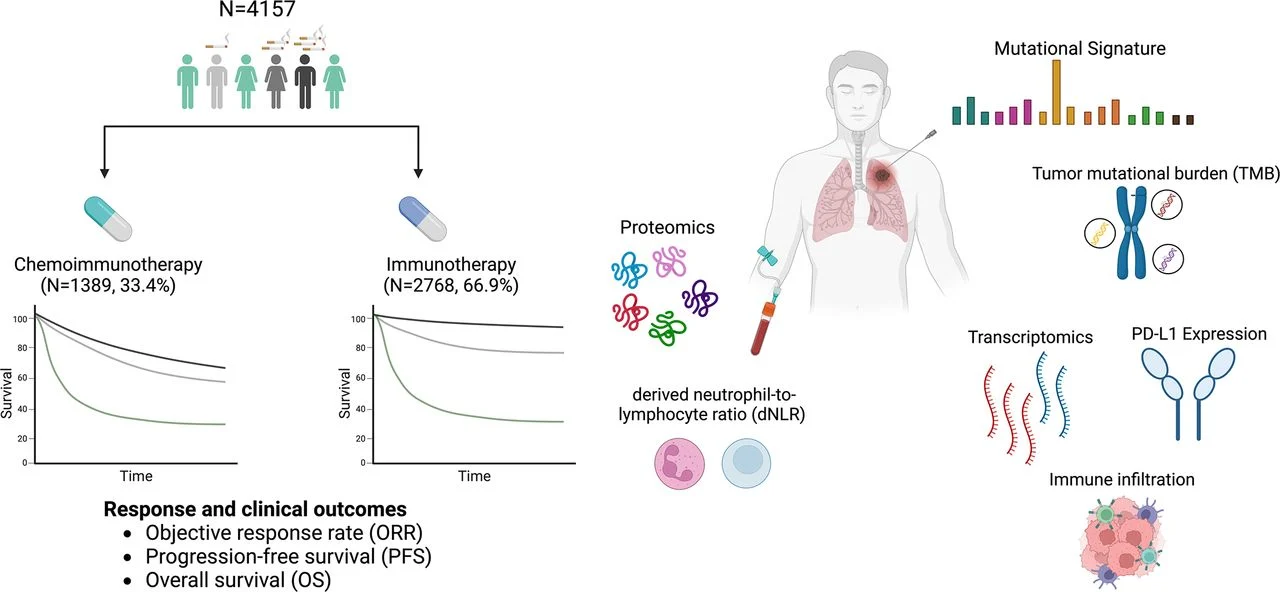
This multi-institutional real-world analysis included 4,157 patients with advanced NSCLC treated at Dana-Farber and MSK (2010–2023): IO alone (n=2,768) or chemo-IO (n=1,389). Smoking was evaluated by status (never/former/current) and cumulative pack-years (light, moderate, heavy). Outcomes (ORR, PFS, OS) were assessed with multivariable models. In PD-L1 TPS ≥50% / EGFR-ALK wild-type, first-line IO alone vs chemo-IO was compared. Mechanistic analyses integrated tumor genomics/transcriptomics, immune infiltration, plasma proteomics, and a tobacco smoking–related mutational signature (TSMS) inferred from panel NGS.
Smoking exposure
Results
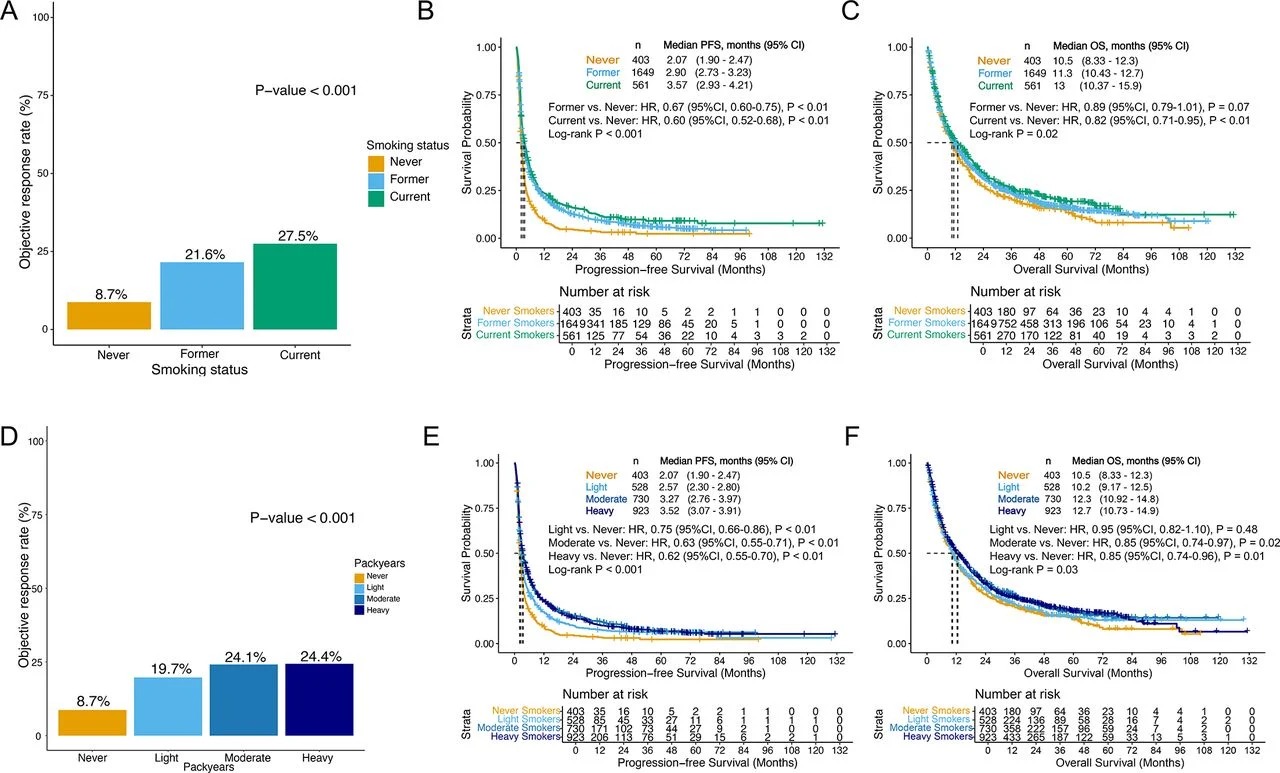
In patients treated with IO alone, smoking history showed a dose-dependent association with improved outcomes: smokers—particularly those with higher pack-years—had higher response rates and longer PFS/OS compared with never-smokers.
In contrast, among patients receiving chemo-IO, smoking history did not meaningfully influence initial response, but current and heavy tobacco exposure were associated with longer PFS and a trend toward improved OS. These relationships remained independent of key co-mutations often linked to smoking biology (including STK11, KEAP1, and KRAS patterns).
A clinically important divergence emerged in the first-line PD-L1 TPS ≥50% / EGFR-ALK WT subgroup. Never-smokers derived clear benefit from chemo-IO over IO alone, with substantially higher response rates and improved PFS (and an OS trend), whereas patients with a smoking history had more comparable long-term outcomes with either strategy.
Key Findings
- IO alone: Smoking exposure (status + pack-years) tracked with better efficacy in a dose-response pattern.
- Chemo-IO: Smoking history did not predict ORR, but current/heavy smokers showed better PFS (and OS trend).
- PD-L1 ≥50% (EGFR/ALK WT): Never-smokers appeared to benefit most from adding chemotherapy up front; smokers showed less separation between IO alone and chemo-IO.
- TSMS derived from routine NGS independently predicted IO-alone benefit (even beyond TMB), providing a molecular proxy when self-reported smoking history is missing or unreliable.
Smoking exposure
Insights
Multi-omics profiling supported a biological basis for these clinical patterns. Tobacco exposure associated with higher TMB, differences in PD-L1 expression, increased tumor-infiltrating lymphocytes, and distinct circulating proteomic immune-signaling profiles. Together, the data suggest that smoking-linked tumor biology may create a more immunogenic context where IO alone can perform well, whereas never-smokers—despite high PD-L1 in some cases—may require chemotherapy-based intensification to maximize early disease control.
Key Takeaway Messages
- Smoking history is not just a demographic variable—capturing it as status + pack-years can inform expected benefit from IO-based strategies.
- In PD-L1–high, EGFR/ALK WT disease, never-smokers may be the subgroup most likely to benefit from first-line chemo-IO rather than IO alone.
- Tobacco mutational signature (TSMS) from standard NGS could function as a practical, stigma-free biomarker when smoking history is unavailable or uncertain.
- These findings are hypothesis-informing for treatment selection and biomarker development, and warrant prospective validation.
Conclusion
Across a large real-world cohort, detailed smoking exposure stratified benefit differently for IO alone versus chemo-IO and highlighted a potentially practice-relevant signal in PD-L1–high disease: never-smokers may preferentially benefit from chemo-IO intensification, while smokers often achieve similar outcomes with IO alone. Integrating clinical smoking metrics with TSMS and multi-omics features may refine immunotherapy selection in advanced NSCLC.
Read all article here