The advent of immunotherapy has transformed the landscape of cancer treatment, offering durable responses in tumors once considered refractory to conventional therapies. Central to this revolution are immune checkpoint inhibitors (ICIs), such as anti–PD-1, PD-L1, and CTLA-4 agents, which unleash the body’s T cells by blocking inhibitory immune signals. Since their initial approvals over a decade ago, ICIs have become foundational in treating a wide range of malignancies—from melanoma to lung and bladder cancers.
More recently, bispecific antibodies (BsAbs) have emerged as a promising new class of immunotherapies. By simultaneously binding tumor antigens and CD3 on T cells, BsAbs redirect immune responses directly to cancer cells, showing efficacy even in tumors with low immune infiltration. Their success in hematologic malignancies has spurred interest in their application to solid tumors.
This article explores and contrasts the mechanisms, clinical efficacy, resistance profiles, toxicity, and future directionsof ICIs and BsAbs. By comparing these non-overlapping approaches, we aim to clarify their respective roles in oncology, discuss opportunities for synergy, and highlight how their evolution is shaping the next era of precision immunotherapy.
Checkpoint Inhibitors vs Bispecific Antibodies in Cancer Therapy
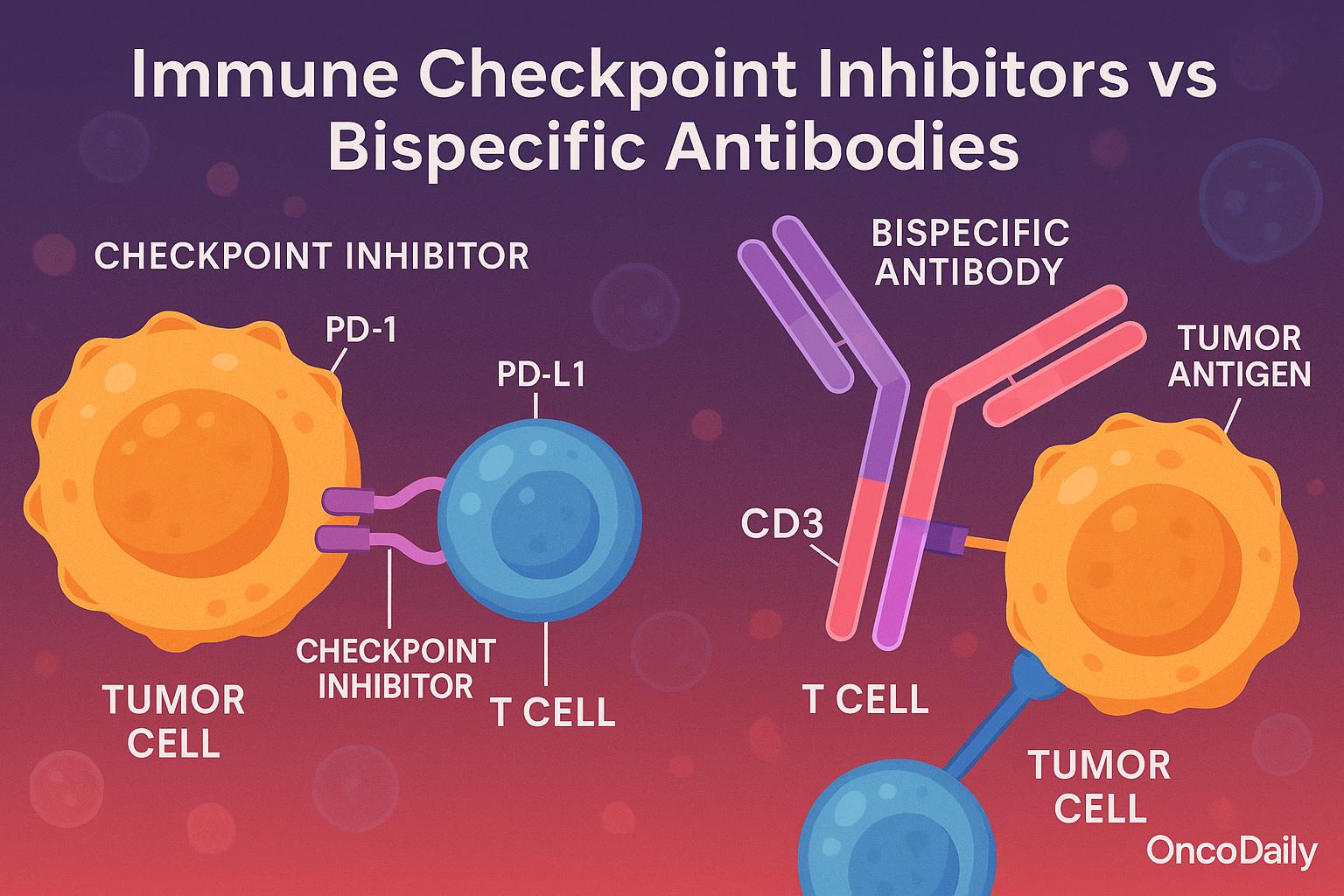
Immune checkpoint inhibitors and bispecific antibodies represent fundamentally different strategies of immune activation in oncology. Checkpoint inhibitors function by lifting inhibitory brakes on T cells, specifically targeting molecules such as PD-1, PD-L1, and CTLA-4. This mechanism restores the natural antitumor activity of pre-existing tumor-infiltrating lymphocytes (TILs), which have often become dysfunctional due to chronic antigen exposure and immune exhaustion. As such, checkpoint blockade is considered a passive form of immune disinhibition that relies on an already primed immune environment. Its efficacy is often limited in immunologically “cold” tumors with sparse T-cell infiltration or low neoantigen burden.
In contrast, bispecific antibodies (BsAbs) offer an active immune engagement approach by physically redirecting immune effector cells—most commonly cytotoxic CD8+ T cells—toward malignant cells. These molecules are engineered to recognize both a tumor-associated antigen (e.g., HER2, CD20, PSMA, CEA) and CD3 on T cells, forming an immune synapse that initiates targeted T-cell activation and tumor lysis independent of antigen presentation via MHC molecules. This attribute gives BsAbs a distinct advantage in tumors that evade immune detection by downregulating MHC or neoantigen expression.
The structural versatility of bispecific antibodies further expands their clinical potential. Formats range from minimal BiTEs (bispecific T-cell engagers), such as blinatumomab, composed of two linked single-chain variable fragments (scFvs), to full-length IgG-like molecules with engineered dual specificity. These IgG-like constructs retain Fc-mediated properties such as extended half-life and effector functions, improving pharmacokinetics and manufacturability. More complex architectures, including trispecific antibodies that bind three targets simultaneously—such as CD38xCD3xCD28—are under investigation to enhance potency, costimulation, and overcome resistance. Collectively, these features enable bispecifics to engage the immune system in a more controlled and modular fashion, with the potential to convert cold tumors into hot, inflamed microenvironments amenable to immune attack.
Immune Activation in Cancer Therapy with Checkpoint Inhibitors and Bispecific Antibodies
Immune checkpoint inhibitors and bispecific antibodies represent fundamentally different strategies of immune activation in oncology. Checkpoint inhibitors function by lifting inhibitory brakes on T cells, specifically targeting molecules such as PD-1, PD-L1, and CTLA-4. This mechanism restores the natural antitumor activity of pre-existing tumor-infiltrating lymphocytes (TILs), which have often become dysfunctional due to chronic antigen exposure and immune exhaustion. As such, checkpoint blockade is considered a passive form of immune disinhibition that relies on an already primed immune environment. Its efficacy is often limited in immunologically “cold” tumors with sparse T-cell infiltration or low neoantigen burden.
In contrast, bispecific antibodies (BsAbs) offer an active immune engagement approach by physically redirecting immune effector cells—most commonly cytotoxic CD8+ T cells—toward malignant cells. These molecules are engineered to recognize both a tumor-associated antigen (e.g., HER2, CD20, PSMA, CEA) and CD3 on T cells, forming an immune synapse that initiates targeted T-cell activation and tumor lysis independent of antigen presentation via MHC molecules. This attribute gives BsAbs a distinct advantage in tumors that evade immune detection by downregulating MHC or neoantigen expression.
The structural versatility of bispecific antibodies further expands their clinical potential. Formats range from minimal BiTEs (bispecific T-cell engagers), such as blinatumomab, composed of two linked single-chain variable fragments (scFvs), to full-length IgG-like molecules with engineered dual specificity. These IgG-like constructs retain Fc-mediated properties such as extended half-life and effector functions, improving pharmacokinetics and manufacturability. More complex architectures, including trispecific antibodies that bind three targets simultaneously—such as CD38xCD3xCD28—are under investigation to enhance potency, costimulation, and overcome resistance. Collectively, these features enable bispecifics to engage the immune system in a more controlled and modular fashion, with the potential to convert cold tumors into hot, inflamed microenvironments amenable to immune attack.
Checkpoint Inhibitors and Bispecific Antibodies in Modern Cancer Therapy
Since the early 2010s, the trajectory of immunotherapy has dramatically reshaped cancer care, beginning with the regulatory success of immune checkpoint inhibitors (ICIs). The first breakthrough came in 2011 with the FDA approval of ipilimumab, a CTLA-4 inhibitor, for advanced melanoma. This paved the way for a wave of PD-1 and PD-L1 inhibitors, such as nivolumab, pembrolizumab, and atezolizumab, which rapidly expanded their indications across a range of malignancies including non-small cell lung cancer (NSCLC), renal cell carcinoma (RCC), urothelial carcinoma, head and neck squamous cell carcinoma (HNSCC), and microsatellite instability-high (MSI-H) tumors, among others. These agents have become a mainstay in both first-line and salvage settings due to their ability to induce durable responses.
In parallel, bispecific antibodies (BsAbs) emerged as a novel platform offering distinct advantages, particularly in hematologic malignancies. The first FDA approval came with blinatumomab, a CD3xCD19 BiTE (bispecific T-cell engager), for acute lymphoblastic leukemia (ALL). More recently, the field has witnessed growing success with mosunetuzumab and glofitamab for follicular lymphoma (FL) and diffuse large B-cell lymphoma (DLBCL). In 2023, epcoritamab—a CD3xCD20 bispecific—received approval for DLBCL, reinforcing the clinical value of this class.
The application of BsAbs in solid tumors is more recent but advancing. Notable approvals include amivantamab, targeting EGFRxMET in EGFR-mutant NSCLC, and tebentafusp, a gp100xCD3 bispecific for uveal melanoma. While promising, BsAbs face distinct challenges compared to ICIs, such as cytokine release syndrome (CRS), need for step-up dosing, and complex administration protocols, often requiring inpatient monitoring during initial cycles.
Despite these hurdles, bispecifics offer a mechanistically unique and potentially synergistic complement to ICIs. As of 2025, their trajectory continues upward, driven by improved engineering, safer formats, and combination strategies with checkpoint inhibitors or cytokine blockers.
Modulating the Tumor Microenvironment with Checkpoint Inhibitors and Bispecific Antibodies
The interaction of immunotherapies with the tumor microenvironment (TME) plays a critical role in determining therapeutic efficacy. Immune checkpoint inhibitors (ICIs), such as PD-1/PD-L1 and CTLA-4 blockers, primarily rely on the presence of pre-existing tumor-infiltrating lymphocytes (TILs) and are most effective in so-called “hot” tumors—those characterized by high PD-L1 expression, high tumor mutational burden (TMB), and an inflamed immune milieu. These tumors already possess some level of immunogenicity, which ICIs amplify by relieving inhibitory signaling on T cells. However, their efficacy is limited in “cold” tumors, where immune infiltration is sparse or absent, rendering the TME resistant to immune activation.
In contrast, bispecific antibodies (BsAbs), particularly those engaging CD3 on T cells and a tumor-associated antigen on cancer cells, actively recruit and redirect immune effectors into the tumor bed. This mechanism enables BsAbs to potentially reshape immune-excluded or immune-desert environments by physically bringing cytotoxic lymphocytes into proximity with malignant cells. As such, BsAbs may have an advantage in overcoming resistance mechanisms rooted in immune exclusion or stromal barriers.
Unlike ICIs, which act independently of specific tumor antigens, BsAbs require the expression of a defined antigen on tumor cells to exert their effects. This antigen dependency introduces challenges in patient selection and resistance due to antigen loss, but it also offers precision in immune targeting. Furthermore, bispecifics can function in MHC-deficient tumors by bypassing the need for antigen presentation, expanding their potential in tumors that evade immune detection through HLA downregulation.
Toxicity Landscape and Management in Checkpoint Inhibitors and Bispecific Antibody Therapies
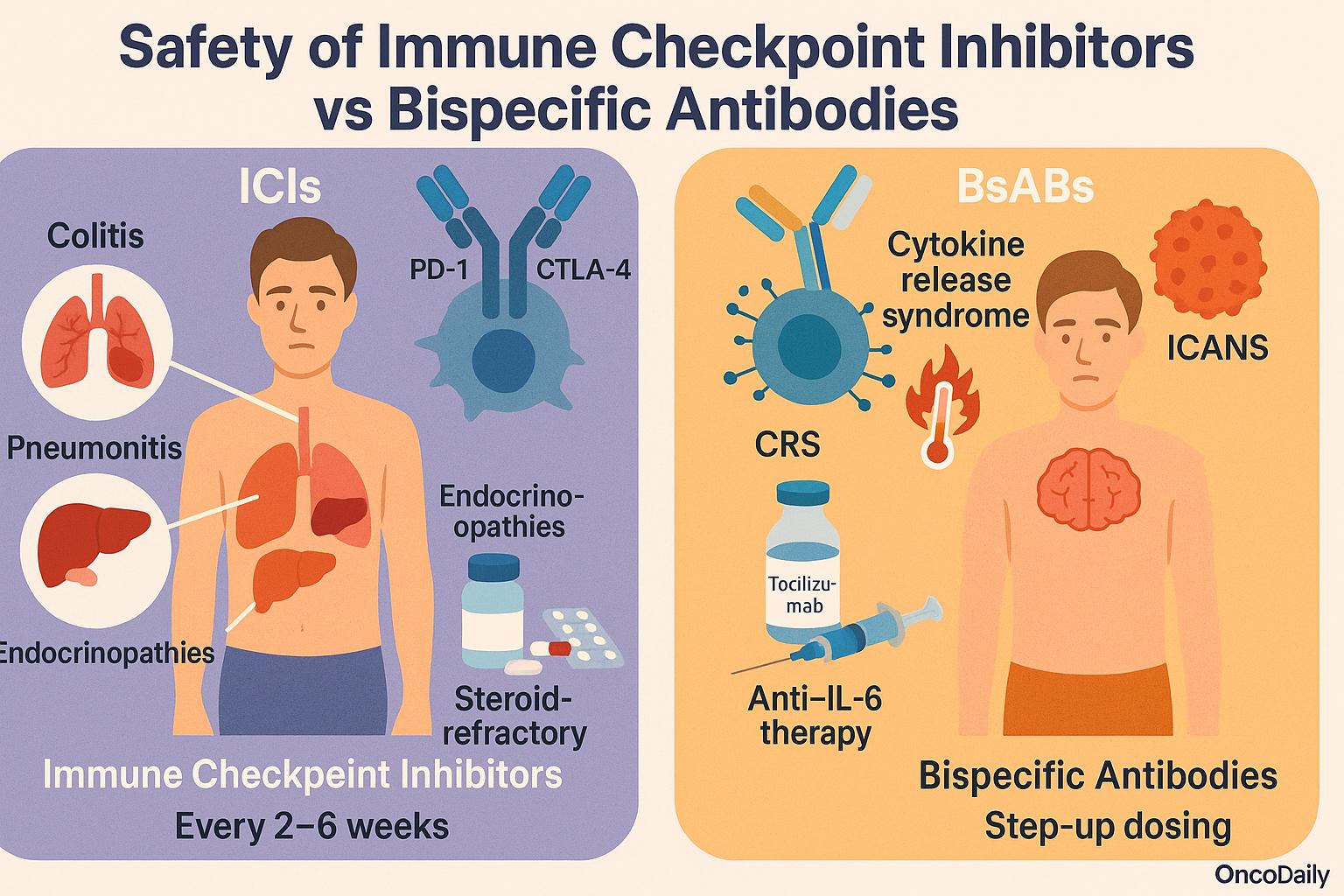
The safety profiles of immune checkpoint inhibitors (ICIs) and bispecific antibodies (BsAbs) differ substantially due to their distinct mechanisms of action. ICIs, such as anti–PD-1, PD-L1, or CTLA-4 agents, unleash pre-existing immune responses and are often associated with immune-related adverse events (irAEs). These toxicities include colitis, pneumonitis, hepatitis, and endocrinopathies, reflecting autoimmunity triggered by systemic immune disinhibition. Management typically involves corticosteroids and, in steroid-refractory cases, other immunosuppressants like infliximab or mycophenolate. ICIs are generally well tolerated long-term and administered every 2–6 weeks in outpatient settings.
In contrast, BsAbs—particularly T cell-engaging molecules such as CD3xCD20 or CD3xBCMA constructs—directly activate and redirect T cells against tumor cells, often irrespective of existing immune priming. This mechanism leads to a higher incidence of acute toxicities, most notably cytokine release syndrome (CRS) and immune effector cell–associated neurotoxicity syndrome (ICANS). These events often occur during initial dosing and may require inpatient monitoring, especially for intravenous formats. Step-up dosing schedules and subcutaneous administration have been developed to mitigate toxicity. CRS is managed with anti–IL-6 therapies like tocilizumab, and ICANS typically responds to corticosteroids.
Resistance Mechanisms and Biomarkers in Immunotherapy
Despite the transformative success of immune checkpoint inhibitors (ICIs) and bispecific antibodies (BsAbs), resistance—both primary and acquired—remains a critical limitation. In the context of ICIs, resistance often arises from impaired antigen presentation, such as mutations in β2-microglobulin (B2M) or loss of major histocompatibility complex (MHC) expression, which renders tumor cells invisible to cytotoxic T cells. Additionally, tumors may develop exclusionary microenvironments that prevent T-cell infiltration or acquire downstream signaling alterations, like JAK1/2 mutations, that impair interferon responsiveness and diminish immune engagement. These mechanisms result in immune escape despite checkpoint inhibition.
BsAbs, by contrast, initiate direct immune synapse formation between T cells and tumor cells, but resistance can still emerge. One mechanism involves downregulation or loss of the target antigen (e.g., CD20, BCMA), which prevents effective T-cell engagement. Moreover, prolonged T-cell activation may lead to functional exhaustion or adaptive resistance, including upregulation of inhibitory checkpoints such as PD-1 or TIM-3, creating a negative feedback loop. These challenges underscore the importance of understanding and anticipating dynamic changes in immune pressure.
Biomarker development is essential to guide therapy and monitor resistance. For ICIs, PD-L1 expression and tumor mutational burden (TMB) remain the most widely studied, though imperfect, predictors. For BsAbs, the expression level of the tumor target (e.g., CD20, HER2) and the functional status and trafficking of CD3+ T cells in the tumor microenvironment are emerging as potential biomarkers of response. Unlike ICIs, BsAbs do not rely on pre-existing T-cell infiltration, but they do require adequate T-cell recruitment and sustained cytotoxic capacity for durable responses.
Importantly, BsAbs may offer a unique advantage in circumventing ICI resistance by forcibly engaging T cells, even in “immune cold” or checkpoint-refractory tumors. This opens the door to synergistic strategies, such as combining BsAbs with ICIs to both initiate and sustain immune pressure while countering adaptive resistance. Ongoing clinical trials are testing such combinations, aiming to exploit the complementary strengths of each modality to achieve deeper and more durable responses.
Future Perspectives and Emerging Strategies in Immuno-Oncology
The future of immunotherapy is increasingly shaped by convergence rather than competition between immune checkpoint inhibitors (ICIs) and bispecific antibodies (BsAbs). These platforms are evolving in parallel, each expanding into novel indications and being re-engineered to address their intrinsic limitations. Rather than replacing one another, they are becoming complementary tools in a growing immunotherapeutic arsenal.
An exciting development is the emergence of checkpoint bispecifics—agents designed to simultaneously block inhibitory pathways and redirect T cells toward tumor antigens. Examples include PD-L1xCD3 and PD-L1xTIGIT constructs, which combine immune disinhibition with direct tumor targeting, potentially enhancing efficacy in resistant or immune-suppressed settings. These dual-function antibodies are currently under early-phase evaluation and may redefine the therapeutic landscape for both hot and cold tumors.
In parallel, next-generation BiTEs are being engineered with extended half-lives, better pharmacokinetics, and optimized CD3 binding to reduce cytokine release syndrome (CRS) and improve outpatient feasibility. Subcutaneous delivery platforms and half-life–extended BiTEs have already shown promise in early clinical trials, allowing broader applicability in solid tumors.
BsAbs are also being explored in minimal residual disease (MRD), adjuvant, and even neoadjuvant settings, where immune pressure may prevent relapse. Their ability to function independently of pre-existing T-cell infiltration makes them especially attractive in early-stage disease or tumors with low immunogenicity. Meanwhile, advances in sequencing and neoantigen prediction open avenues for personalized BsAbs that target patient-specific tumor mutations—a highly tailored and potentially more effective therapeutic strategy.
Combination regimens are central to ongoing research. Numerous trials are evaluating BsAbs alongside ICIs, cancer vaccines, and antibody-drug conjugates (ADCs), aiming to overcome resistance and enhance immunologic synergy. The immunotherapy pipeline is rapidly expanding, as highlighted in recent meetings like ASCO, ESMO, and AACR 2024–2025, with a wave of innovative constructs targeting HER2, PSMA, GPRC5D, CEACAM5, and beyond.
You Can Watch More on OncoDaily Youtube TV
FAQ
What are checkpoint inhibitors in cancer therapy?
Checkpoint inhibitors are immunotherapy drugs that block proteins like PD-1, PD-L1, or CTLA-4 to release the "brakes" on T cells, enabling them to attack cancer cells more effectively.
How do bispecific antibodies work in immunotherapy?
Bispecific antibodies bind both a tumor antigen and CD3 on T cells, physically redirecting the immune response toward cancer cells—regardless of pre-existing T-cell activity.
Which is more effective: checkpoint inhibitors or bispecific antibodies?
Effectiveness depends on tumor type and immune context. ICIs work well in “hot” tumors with T-cell infiltration, while BsAbs may be superior in “cold” or MHC-deficient tumors.
What cancers are treated with checkpoint inhibitors?
ICIs are approved for melanoma, lung, kidney, bladder, head and neck cancers, and MSI-high tumors, among others.
Are bispecific antibodies approved for solid tumors?
Yes. While initially used for blood cancers, recent approvals include amivantamab (NSCLC) and tebentafusp (uveal melanoma). Many others are in clinical trials.
What are the side effects of bispecific antibodies?
Common toxicities include cytokine release syndrome (CRS) and neurotoxicity (ICANS), typically during initial dosing. Step-up dosing and tocilizumab help manage these effects.
Can bispecific antibodies overcome ICI resistance?
Yes. BsAbs can activate T cells independently of antigen presentation or immune priming, making them effective even in tumors resistant to checkpoint blockade.
What is the difference between BiTEs and full-length BsAbs?
BiTEs are small, short-acting molecules, while full-length BsAbs resemble IgG antibodies, offering longer half-life, better stability, and additional immune functions.
How does the tumor microenvironment affect immunotherapy response?
Hot tumors with T-cell infiltration respond well to ICIs. Cold or immune-excluded tumors may resist ICIs but can respond to BsAbs that actively bring T cells into the tumor.
Are checkpoint inhibitors and BsAbs used together?
Yes. Ongoing trials are testing combinations to enhance immune activation and overcome resistance. Dual-function bispecifics combining checkpoint blockade with T-cell engagement are in development.