The ROSELLA trial (GOG-3073/ENGOT-ov72) is a phase 3, international study designed to evaluate the efficacy of relacorilant—a selective glucocorticoid receptor antagonist—combined with nab-paclitaxel in patients with platinum-resistant ovarian cancer. This disease state is associated with poor outcomes, particularly after progression on platinum-based regimens, bevacizumab, and PARP inhibitors. Standard non-platinum chemotherapy, such as weekly taxanes, offers limited benefit, with median progression-free survival typically under six months. Given the role of glucocorticoid signaling in mediating chemotherapy resistance, ROSELLA aimed to determine whether targeting this pathway with relacorilant could enhance the anti-tumor activity of nab-paclitaxel and improve survival outcomes in this heavily pretreated population.
Tite:Relacorilant and nab-paclitaxel in patients with platinum-resistant ovarian cancer (ROSELLA): an open-label, randomised, controlled, phase 3 trial
Authors: Alexander B Olawaiye, MD, Laurence Gladieff, MD, David M O’Malley, MD, Prof Jae-Weon Kim, MD, Gabriel Garbaos, MD, Vanda Salutari, MD, Lucy Gilbert, MD, Linda Mileshkin, MBBS, Prof Alix Devaux, MD, Elizabeth Hopp, MD, Prof Yong Jae Lee, MD, Prof Ana Oaknin, MD, Mariana Scaranti, MD, Prof Byoung-Gie Kim, MD, Prof Nicoletta Colombo, MD, Michael E McCollum, MD, Connie Diakos, MBBS, Andrew Clam, BMBCh, Aliza L Leiser, MD, Boglárka Balázs, MD, Prof Bradley J Monk, MD, Giuseppa Scandurra, MD, Emily McClung, MD, Emilie Kaczmarek, MD, Brian Slomovitz, MD, Helena De La Cueva, MD, Aknar Freire de Carvalho Calabrich, MD, Chiara Cassani, MD, Prof Benoit You, MD, Prof Toon Van Gorp, MD, Cristina Churruca, MD, Prof Giuseppe Caruso, MD, Shibani Nicum, MB, Andrea Bagaméri, MD, Grazia Artioli, MD, Lubomir Bodnar, MD , Prof Sokbom Kang, MD, Prof Ignace Vergote, MD, Amanda Kesner-Hays, PhD, Hristina I Pashova, PhD, Sachin G Pai, MD, Iulia Cristina Tudor, PhD, Adrian M Jubb, FRCPathat ∙ Prof Domenica Lorusso, MD
Published in Lancet Oncology, June 2025
Background
Platinum-resistant ovarian cancer presents a significant clinical challenge, with limited treatment options and poor survival outcomes. While weekly taxane-based chemotherapy remains a key treatment approach, outcomes remain suboptimal, particularly following prior exposure to bevacizumab and poly (ADP-ribose) polymerase (PARP) inhibitors. Glucocorticoid receptor signaling is known to impair chemotherapy efficacy by upregulating anti-apoptotic pathways, a mechanism targeted by relacorilant—a novel, selective glucocorticoid receptor antagonist. The phase 3 ROSELLA trial aimed to evaluate whether combining relacorilant with nab-paclitaxel could improve progression-free survival (PFS) and overall survival (OS) in women with platinum-resistant ovarian cancer.
Methods and Stdy Design
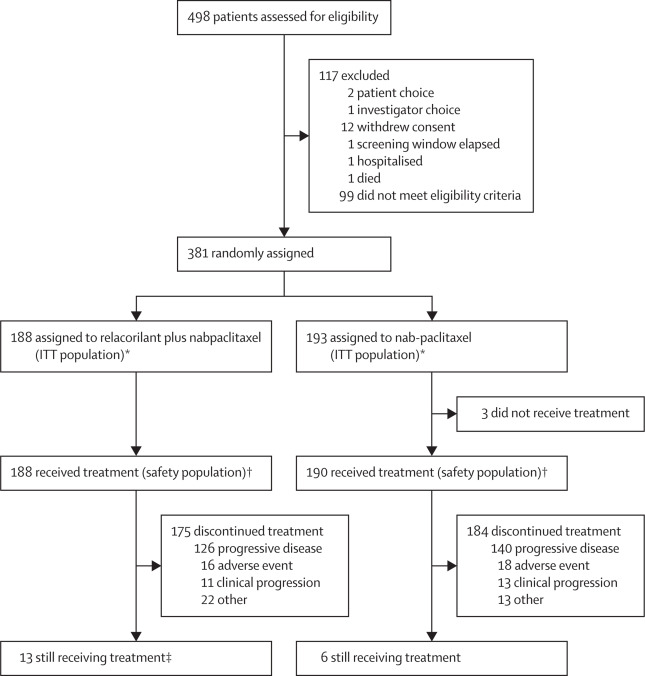
The ROSELLA trial (GOG-3073/ENGOT-ov72; NCT05257408) was a randomized, controlled, open-label, phase 3 study conducted across 117 sites in 14 countries. Eligible patients were adult females (≥18 years) with platinum-resistant epithelial ovarian, primary peritoneal, or fallopian tube cancers who had received one to three prior lines of systemic therapy, including bevacizumab, and demonstrated disease progression or intolerance to their last regimen.
Participants were randomized 1:1 to receive either:
- Relacorilant (150 mg orally) the day before, the day of, and the day after nab-paclitaxel (80 mg/m² IV) on days 1, 8, and 15 of a 28-day cycle
- Nab-paclitaxel monotherapy (100 mg/m² IV) on the same schedule.
Dual primary endpoints were PFS (by blinded independent central review, BICR) and OS, analyzed in the intent-to-treat population.
A total of 381 patients were enrolled between January 2023 and April 2024. Patients were stratified by region and number of prior treatment lines. Crossover was not allowed, and the study included robust sensitivity analyses and exposure-adjusted safety assessments. The study’s power was set at 86.4% to detect a 50% improvement in PFS (HR 0.66), requiring 230 events with a two-sided α=0.04. Interim OS analysis was planned at the time of PFS assessment.
Results
Among 381 patients randomized (188 to combination, 193 to monotherapy), all had prior taxane and bevacizumab exposure, and 61% had prior PARP inhibitor therapy. PFS by BICR was significantly longer in the combination group:
- Median PFS: 6.54 months (95% CI 5.55–7.43) vs 5.52 months (3.94–5.88)
- HR: 0.70 (95% CI 0.54–0.91), p=0.0076
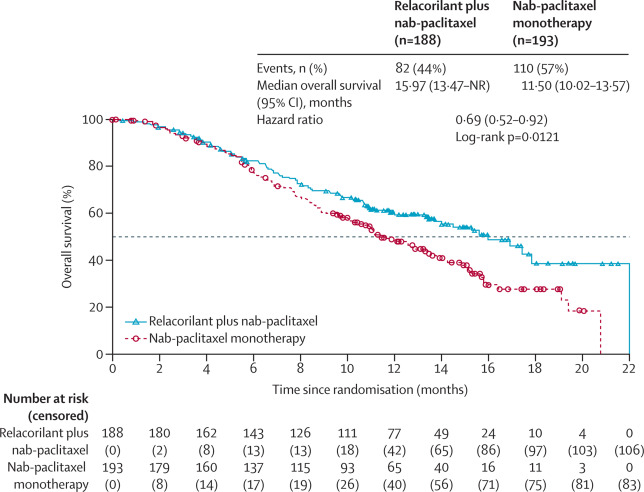
At interim OS analysis:
- Median OS: 15.97 months (13.47–NR) vs 11.50 months (10.02–13.57)
- HR: 0.69 (95% CI 0.52–0.92), p=0.0121
- 12-month survival: 60% vs 49%
Investigator-assessed PFS (HR 0.71, p=0.0030) and clinical benefit rate at 24 weeks (51.1% vs 38.9%, p=0.016) also favored the combination. The objective response rate was numerically higher in the relacorilant arm (36.9% vs 30.1%).
Key Findings
The ROSELLA trial confirms the clinical benefit of relacorilant plus nab-paclitaxel in platinum-resistant ovarian cancer, meeting both primary endpoints. The observed improvement in survival outcomes, combined with a manageable safety profile, supports the combination as a promising new treatment option. These findings also underscore the potential of targeting glucocorticoid receptor signaling in overcoming chemoresistance in ovarian cancer and beyond.
- Relacorilant plus nab-paclitaxel demonstrated statistically significant and clinically meaningful improvement in PFS and interim OS.
- Benefits were consistent across subgroups, including those with poor prognostic factors (e.g., older age, high disease burden, short platinum-free interval).
- Median PFS gain of ~1 month and OS gain of ~4.5 months compared to nab-paclitaxel alone.
- Safety profile was manageable and consistent with prior data; no new signals observed.
Most common grade ≥3 adverse events were neutropenia (44% vs 25%), anemia (18% vs 8%), and fatigue (9% vs 2%), corresponding with longer exposure in the combination group.
Key Takeaway Messages
- The ROSELLA trial is the first registrational study to demonstrate the efficacy of a selective glucocorticoid receptor antagonist in cancer.
- Relacorilant enhances chemotherapy sensitivity without requiring biomarker selection.
- The combination therapy has a favorable benefit-risk profile and represents a potential new standard of care for platinum-resistant ovarian cancer.
- Future studies may explore triplet regimens including bevacizumab, and use of relacorilant in other solid tumors.
You can read the full rticle here
Written by Sona Karamyan, MD


