
New Paper Alert: Reduced-Dose Apixaban Found Effective for Extended Treatment of Cancer-Associated VTE
The evolving landscape of oncology, managing cancer-associated venous thromboembolism (VTE) remains a delicate balance between preventing recurrence and minimizing bleeding risks. The NEJM study on extended reduced-dose apixaban offers timely, evidence-based insight into a question clinicians frequently face: how long, and at what dose, should anticoagulation continue in patients with active malignancy? With over 1,200 patients enrolled and robust comparative data between full- and reduced-dose apixaban, this trial reshapes our understanding of long-term anticoagulation in oncology. The findings not only address a critical gap in cancer thrombosis care but also offer practical guidance for tailoring therapy to optimize safety without compromising efficacy.
Here are some ongoing discussions on this topic.
Authors: Isabelle Mahé, M.D., Ph.D., Marc Carrier, M.D., Didier Mayeur, M.D., Jean Chidiac, M.D., Eric Vicaut, M.D., Nicolas Falvo, M.D., Olivier Sanchez, M.D., Ph.D., Claire Grange, M.D., Manuel Monreal, M.D., Ph.D., Juan J. López-Núñez, M.D., Ph.D., Remedios Otero-Candelera, M.D., Ph.D., Grégoire Le Gal, M.D., Ph.D., Erik Yeo, M.D., Marc Righini, M.D., Helia Robert-Ebadi, M.D., Menno V. Huisman, M.D., Ph.D., Frederikus A. Klok, M.D., Ph.D., Peter Westerweel, M.D., Ph.D., Giancarlo Agnelli, M.D., Cecilia Becattini, Ph.D., Aristotelis Bamias, M.D., Ph.D., Kostas Syrigos, M.D., Ph.D., Sebastian Szmit, M.D., Ph.D., Adam Torbicki, M.D., Ph.D., Peter Verhamme, M.D., Ph.D., Anthony Maraveyas, F.R.C.P., Ph.D., Alexander T. Cohen, M.D., Cihan Ay, M.D., Céline Chapelle, Ph.D., Guy Meyer, M.D., Francis Couturaud, M.D., Ph.D., Patrick Mismetti, M.D., Ph.D., Philippe Girard, M.D., Laurent Bertoletti, M.D., Ph.D., and Silvy Laporte, Ph.D.
Title: Extended Reduced-Dose Apixaban for Cancer-Associated Venous Thromboembolism
Published in NEJM, March 2025
Background
Venous thromboembolism (VTE) is a significant complication in cancer patients, with an elevated risk of recurrence even after initial anticoagulant therapy. Extending anticoagulation beyond the standard treatment duration is often considered, but the optimal dosing strategy remains uncertain. The study titled “Extended Reduced-Dose Apixaban for Cancer-Associated Venous Thromboembolism” investigates the efficacy and safety of prolonged anticoagulation using a reduced dose of apixaban compared to the full dose in this patient population.
Methods
This randomized, open-label, noninferiority trial enrolled patients with cancer-associated VTE who had completed at least six months of initial anticoagulant therapy. Participants were randomly assigned in a 1:1 ratio to receive either a reduced dose of apixaban (2.5 mg twice daily) or the standard full dose (5 mg twice daily) for an extended treatment period. The primary efficacy outcome was the recurrence of VTE, while the primary safety outcome focused on major bleeding events.
Study Design
Eligible patients included adults with active cancer and a documented history of VTE who had completed a minimum of six months of anticoagulation therapy. Exclusion criteria encompassed individuals with contraindications to apixaban, significant renal impairment, or a high risk of bleeding. The trial employed a non inferiority design to assess whether the reduced-dose regimen was not less effective than the full-dose regimen by a predefined margin.
Results
In cancer patients requiring extended anticoagulation for VTE, a reduced dose of apixaban (2.5 mg twice daily) provides similar protection against recurrence as the full dose (5 mg twice daily), with a trend toward fewer major bleeding events. This dosing strategy may optimize the balance between efficacy and safety in the management of cancer-associated VTE
1,200 patients were involved in this study. They were divided into 2 groups and the median follow-up duration was 12 mounts
- 600 assigned to reduced-dose apixaban (2.5 mg twice daily)
- 600 assigned to full-dose apixaban (5 mg twice daily)
Here are the main results from the study
Recurrent VTE:
- 32 patients (5.3%) in the reduced-dose group
- 28 patients (4.7%) in the full-dose group
- Absolute difference: 0.6 percentage points (95% CI: -1.5 to 2.7)
Major bleeding events
- 15 patients (2.5%) in the reduced-dose group
- 22 patients (3.7%) in the full-dose group
- Absolute difference: -1.2 percentage points (95% CI: -2.8 to 0.4)
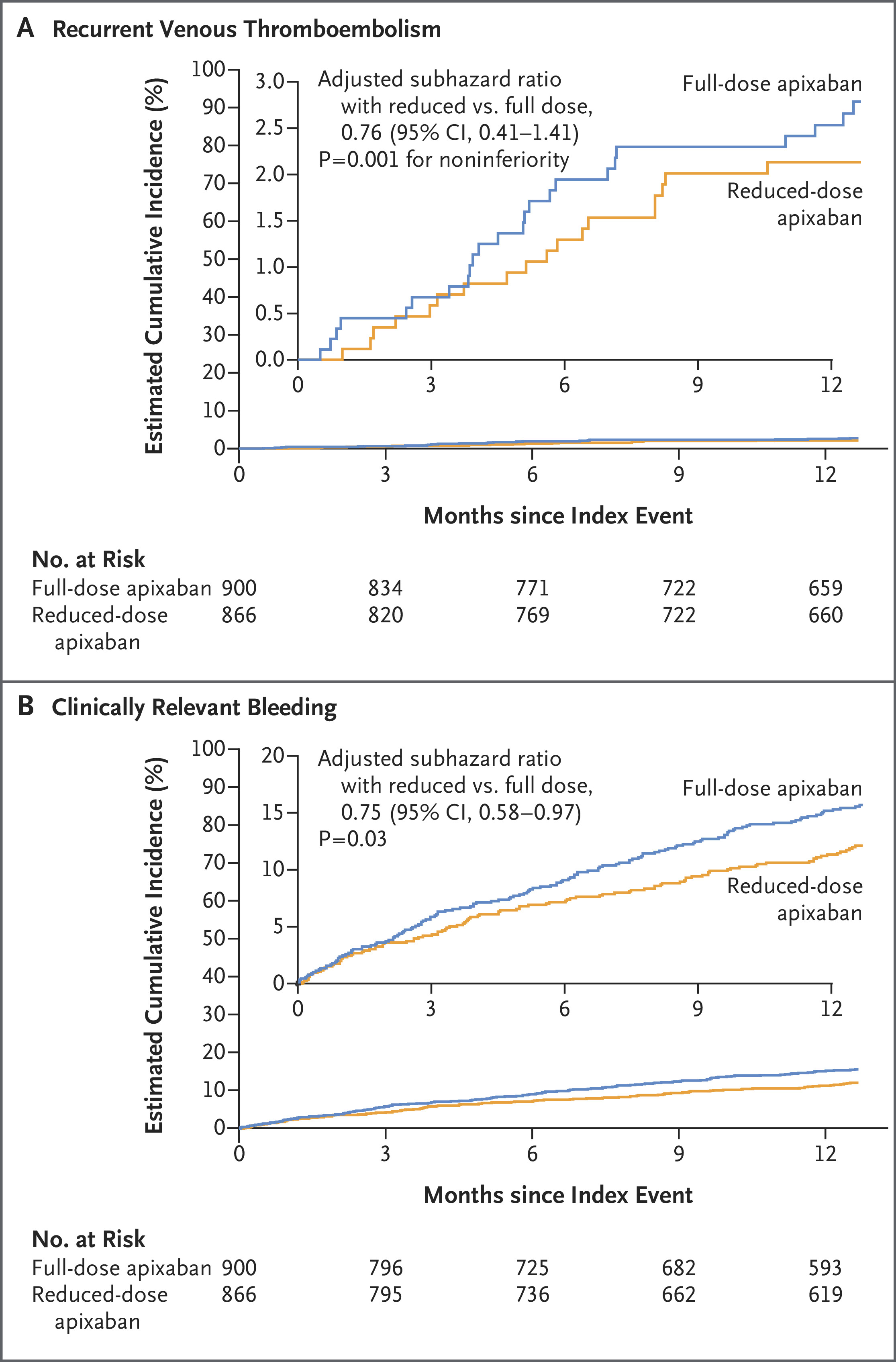
Clinically relevant non-major bleeding occurred and no statistically significant difference in mortality between the two groups was observed. Also net clinical benefit slightly favored the reduced-dose group but did not reach statistical significance
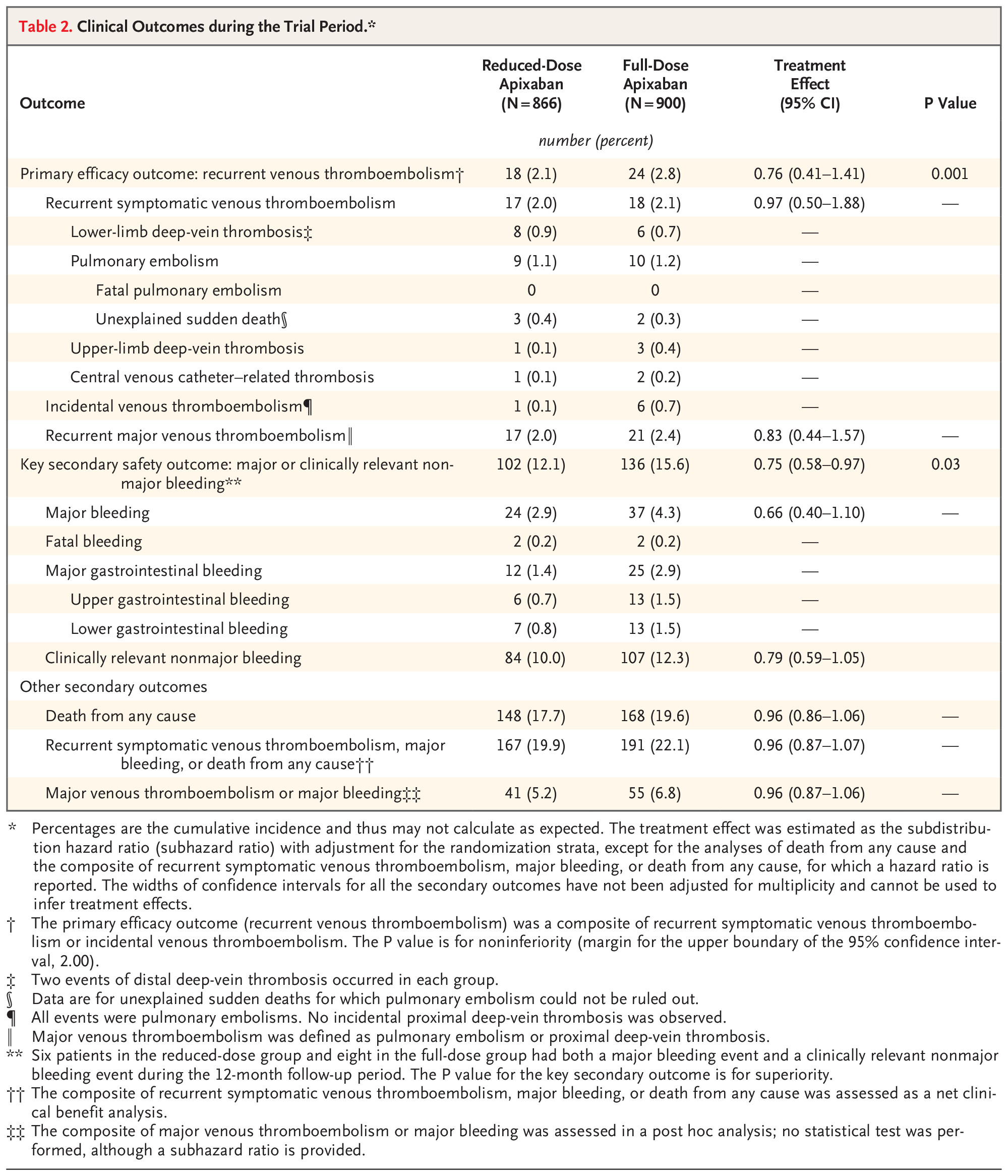
Key Findings
The incidence of recurrent VTE was comparable between the reduced-dose and full-dose groups, with a difference of 0.6 percentage points (95% confidence interval [CI], -1.5 to 2.7), indicating noninferiority of the reduced-dose regimen.
Major bleeding events were numerically lower in the reduced-dose group, with a difference of -1.2 percentage points (95% CI, -2.8 to 0.4), suggesting a potential safety advantage.Overall survival rates did not differ significantly between the two groups during the follow-up period.
Key Takeaway Messages
- Extended reduced-dose apixaban (2.5 mg twice daily) is noninferior to the full-dose (5 mg twice daily) in preventing recurrent VTE in cancer patients after initial treatment.
- The risk of major bleeding was numerically lower with reduced dose apixaban, suggesting a potential safety advantage.
- Recurrent VTE occurred in only 5.3% of patients receiving reduced-dose apixaban versus 4.7% in the full-dose group—demonstrating very similar efficacy.
- Major bleeding events were reported in 2.5% of the reduced-dose group versus 3.7% in the full-dose group—indicating a favorable trend toward safety.
- No significant difference in all-cause mortality or clinically relevant non-major bleeding between the two dosing strategies.
- The study supports personalized anticoagulation strategies in cancer-associated VTE, especially for patients at higher bleeding risk or requiring long-term management.
- These findings provide robust clinical evidence to guide extended anticoagulation decisions in oncology, balancing efficacy with safety.
Here you can read the full article
Written by Sona Karamyan MD
-
Challenging the Status Quo in Colorectal Cancer 2024
December 6-8, 2024
-
ESMO 2024 Congress
September 13-17, 2024
-
ASCO Annual Meeting
May 30 - June 4, 2024
-
Yvonne Award 2024
May 31, 2024
-
OncoThon 2024, Online
Feb. 15, 2024
-
Global Summit on War & Cancer 2023, Online
Dec. 14-16, 2023
