Peri-operative immunotherapy combined with platinum-based neoadjuvant chemotherapy has become a key treatment strategy in resectable stage II-IIIA NSCLC. While CheckMate-816 and KEYNOTE-671 demonstrated clinical benefit with nivolumab/pembrolizumab-based regimens, RATIONALE-315 is the first large phase III study to evaluate peri-operative tislelizumab in this setting, including both neoadjuvant and adjuvant phases.
Study Design
RATIONALE-315 was a phase III, randomized, double-blind, placebo-controlled trial conducted across 50 centers in China. A total of 453 patients with resectable stage II–IIIA non-small cell lung cancer (NSCLC) were enrolled and randomly assigned in a 1:1 ratio to receive either tislelizumab plus neoadjuvant chemotherapy (n=226) or placebo plus neoadjuvant chemotherapy (n=227).
All patients were treatment-naïve, had an ECOG performance status of 0–1, and were considered suitable for surgical resection with curative intent. Importantly, patients with EGFR mutations or ALK rearrangements were not eligible, ensuring a population representative of immunotherapy-responsive NSCLC.
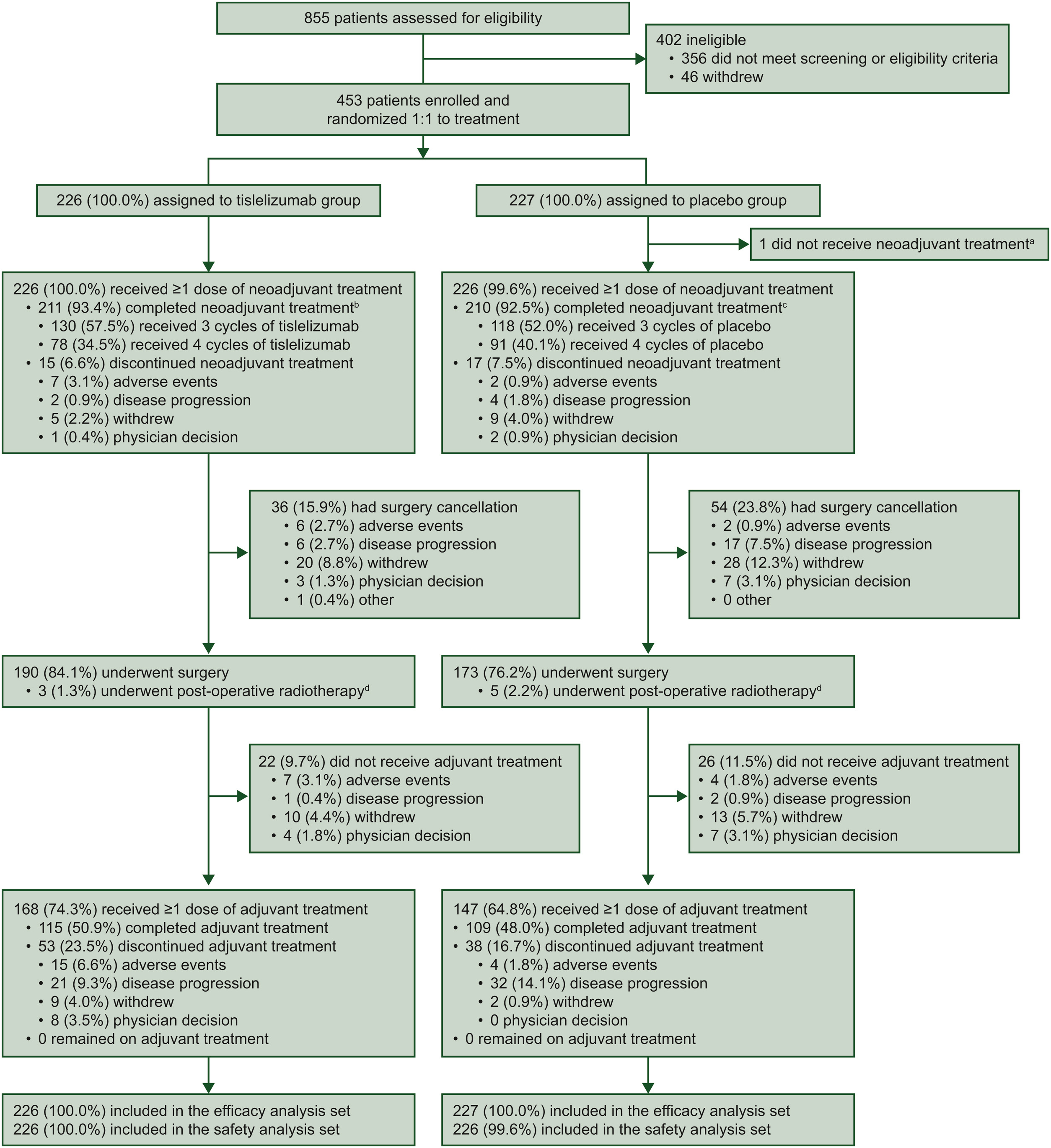
RATIONALE-315
Treatment Protocol
Patients first received neoadjuvant therapy, consisting of 3–4 cycles every 3 weeks. In this phase, they were treated with either tislelizumab 200 mg or placebo, combined with platinum-based doublet chemotherapy. Chemotherapy was tailored to tumor histology:
• Cisplatin or carboplatin plus paclitaxel for squamous NSCLC
• Cisplatin or carboplatin plus pemetrexed for non-squamous NSCLC
Following neoadjuvant treatment, patients proceeded to curative-intent surgery (R0 planned) typically 4–6 weeks later.
After recovery from surgery, eligible patients began adjuvant treatment, receiving tislelizumab 400 mg or placebo every 6 weeks for up to eight cycles. Postoperative radiotherapy was allowed in cases with positive surgical margins or N2 nodal involvement, reflecting real-world high-risk management.
The trial evaluated major pathological response (MPR) and event-free survival (EFS) as co-primary endpoints. Key secondary outcomes included pathological complete response (pCR), overall survival (OS), disease-free survival (DFS), and safety.
Results (Final Analysis, Median Follow-Up 38.5 Months)
At the time of final analysis, peri-operative tislelizumab combined with platinum-based neoadjuvant chemotherapy demonstrated significant survival benefit vs placebo.
Overall Survival
Treatment with tislelizumab resulted in a 35% reduction in risk of death:
- HR 0.65 (95% CI 0.45–0.93), P=0.009
- Median OS: Not reached in both arms
36-month OS rate
- Tislelizumab: 79.3%
- Placebo: 69.3%
The OS curves began to diverge from approximately month 9 and remained separated long-term, indicating durable benefit.
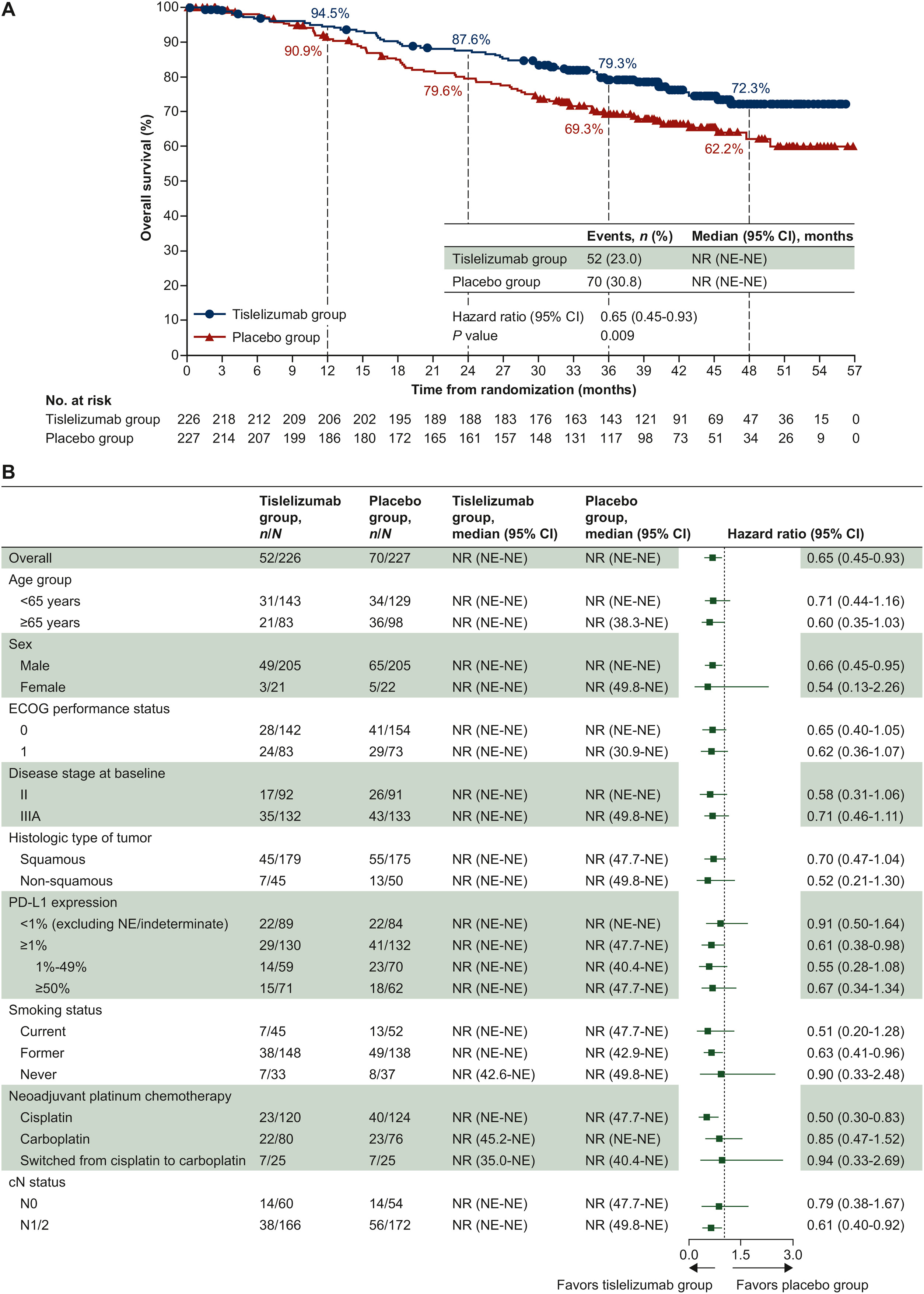
RATIONALE-315
Event-Free Survival
Event-free survival outcomes favored the tislelizumab arm:
- Median EFS: Not reached vs 30.6 months for placebo
- HR 0.58 (95% CI 0.43–0.79)
36-month EFS rate
- Tislelizumab: 64.7%
- Placebo: 48.0%
EFS curves separated early (<3 months) and remained distinctly apart, suggesting strong early tumor response and durable disease control.
Pathological Response (from earlier interim report)
Neoadjuvant tislelizumab led to substantially higher pathological response rates:
- Major pathological response (MPR): 56.2% vs 15.0% (P < 0.0001)
- pCR also significantly improved, reinforcing deep tumor regression pre-surgery
Subgroup Consistency
Benefit observed regardless of:
- Stage II or IIIA
- Squamous or non-squamous histology
- PD-L1 <1% or ≥1% (stronger effect in PD-L1 ≥1%)
- Smoking status and nodal status
Biomarker Analysis
Exploratory evaluation of tumor mutational burden (TMB) revealed no meaningful differences in overall survival between TMB-high and TMB-low subgroups. Median OS was not reached in either group for patients receiving tislelizumab or placebo, and Kaplan–Meier curves showed no separation. These findings suggest that, unlike advanced-stage disease settings where TMB occasionally correlates with immunotherapy response, TMB does not appear to function as a predictive biomarker for peri-operative PD-1 blockade in early-stage resectable NSCLC. Therefore, its utility for patient selection in this context remains limited, highlighting the ongoing need for more refined biomarkers.
Safety Profile
Safety outcomes were generally consistent with expected toxicities of platinum chemotherapy combined with PD-1 inhibition. Nearly all patients in both arms experienced treatment-related adverse events, reflecting chemotherapy-driven toxicity:
- TRAEs occurred in 99.1% of patients in the tislelizumab arm and 99.6% in the placebo arm.
- Grade ≥3 TRAEs were slightly higher with tislelizumab (73.0% vs 67.3%), and serious treatment-related events were also more frequent (15.5% vs 8.8%).
- Immune-mediated events, as expected with checkpoint blockade, occurred more often in the tislelizumab group, most commonly:
- Cutaneous toxicities (17.7%)
- Hypothyroidism (14.6%)
- Pneumonitis (8.0%)
Treatment-related mortality was low in both arms (1.8% with tislelizumab vs 0.9% with placebo) and no unexpected safety signals emerged. Overall, despite a higher rate of immune-mediated toxicity, the regimen was considered manageable, aligning with known safety characteristics of anti–PD-1 therapy.
You can read the Article here


