RATIONALE-305 Trial, fresh from the ASCO Gastrointestinal Cancers Symposium, delivered a post hoc analysis examining how clinician-rated ECOG performance status (0 vs 1) aligns with patient-reported outcomes (PROs) at baseline in patients with first-line locally advanced or metastatic gastric or gastroesophageal junction adenocarcinoma (GC/GEJC). The analysis explored concordance between traditional performance status assessment and patient-reported symptom burden and functioning at treatment initiation.
Background
ECOG performance status is routinely used to guide eligibility, stratification, and prognostic assessment in clinical trials and everyday oncology practice. It plays a central role in treatment decision-making, trial enrollment, and interpretation of outcomes across solid tumors, including advanced gastric and gastroesophageal junction cancers. However, as a clinician-rated measure, ECOG-PS is subject to interobserver variability and relies on brief clinical assessment, which may not fully capture the multidimensional symptom burden, functional limitations, and quality-of-life impairments experienced by patients.
Patient-reported outcomes provide a complementary perspective by directly capturing patients’ perceptions of symptoms, daily functioning, and overall well-being. In gastric and gastroesophageal junction cancer, disease-related symptoms such as pain, fatigue, gastrointestinal dysfunction, and nutritional limitations can significantly affect daily life, even when clinician-rated performance status appears relatively preserved. Understanding how ECOG-PS corresponds to baseline PROs may help clarify the extent to which traditional performance status reflects the patient experience at treatment start.
In this context, the present analysis examined whether baseline ECOG-PS categories meaningfully distinguish patient-reported symptom burden and functional status at treatment initiation, independent of assigned treatment arm, thereby assessing the extent to which ECOG-PS aligns with the patient experience in advanced gastric and gastroesophageal junction cancer.
Study design
RATIONALE-305 was a randomized, double-blind, phase 3 trial in 1L unresectable locally advanced or metastatic GC/GEJC (HER2-negative; ECOG 0–1), comparing tislelizumab + chemotherapy vs placebo + chemotherapy, with optional capecitabine maintenance later in treatment.
Methods
Among 932 randomized patients with completed baseline PRO assessments, investigators evaluated patient-reported outcomes using the EORTC QLQ-C30 and the gastric cancer–specific EORTC QLQ-STO22 questionnaires. Baseline PRO scores were compared between patients with ECOG-PS 0 and ECOG-PS 1, pooling data across treatment arms. Multivariable regression models were also applied to examine associations between ECOG performance status and individual PRO domains.
Results of RATIONALE-305 Trial
Patients with baseline ECOG-PS 1 reported significantly worse patient-reported outcomes compared with those with ECOG-PS 0, including lower global health status/quality of life (68.0 vs 72.5; P < 0.001), reduced physical functioning (87.6 vs 89.8; P = 0.024), and higher pain burden (80.3 vs 83.5; P = 0.034).
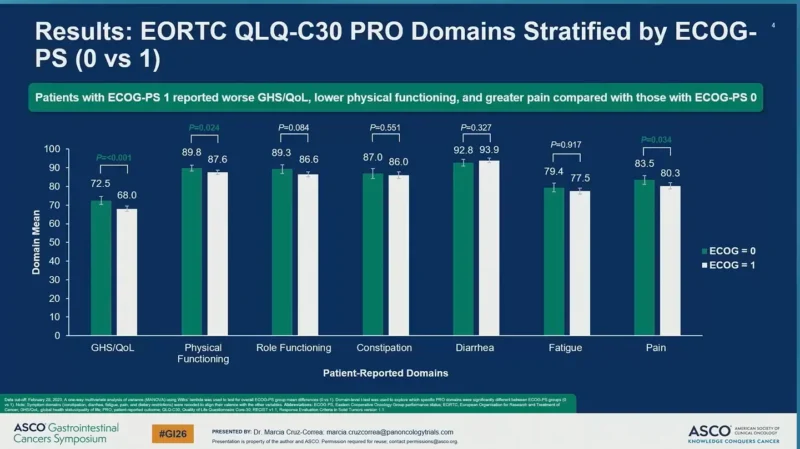
Role functioning (86.6 vs 89.3; P = 0.084), constipation (86.0 vs 87.0; P = 0.551), diarrhea (93.9 vs 92.8; P = 0.327), and fatigue (77.5 vs 79.4; P = 0.917) were reported for patients with ECOG-PS 1 compared with ECOG-PS 0, respectively.
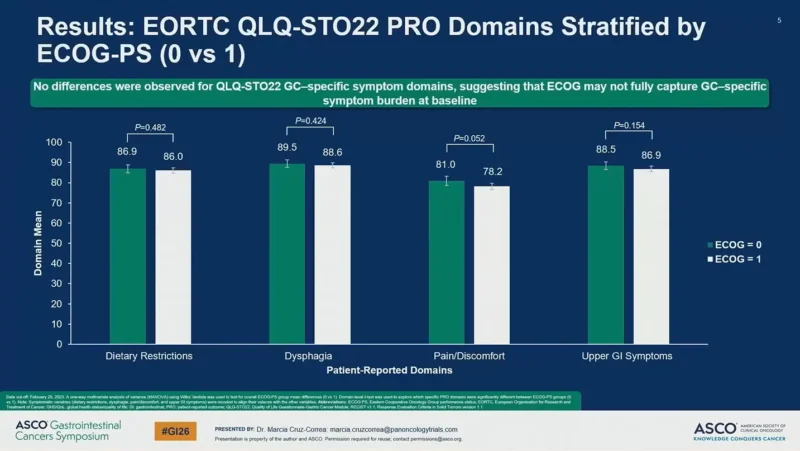
QLQ-STO22 (gastric cancer–specific symptoms)
No statistically significant baseline differences were seen between ECOG 0 vs 1 for:
- Dietary restrictions (P = 0.482)
- Dysphagia (P = 0.424)
- Upper GI symptoms (P = 0.154)
- Pain/discomfort (P = 0.052).
Key takeaways
ECOG-PS does capture part of the baseline patient experience in advanced GC/GEJC—especially overall QoL, physical functioning, and pain—but it did not differentiate key GC-specific symptom domains on STO22 at baseline. The message is practical: baseline PROs can add important nuance beyond ECOG when assessing patients and designing trials.
Conclusion
This post hoc analysis from RATIONALE-305 provides a detailed baseline comparison of clinician-rated performance status and patient-reported outcomes in first-line advanced GC/GEJC. By systematically evaluating both general and gastric cancer–specific PRO instruments alongside ECOG-PS, the analysis offers a comprehensive snapshot of patient-reported symptom burden and functioning at treatment initiation. These findings support continued exploration of how clinician assessments and patient-reported measures can be integrated to inform trial design, baseline characterization, and longitudinal evaluation in advanced gastric and gastroesophageal junction cancer.
What to watch next
Ongoing work from RATIONALE-305 is evaluating whether ECOG-PS meaningfully differentiates PRO trajectories over time and whether combining ECOG + PROs can improve risk stratification and patient-centered trial design.
For more information click here.


