Original Study Title: DAHANCA19: A randomized phase III study of primary curative (chemo)-radiotherapy and the EGFR-inhibitor zalutumumab for squamous cell carcinoma of the head and neck
Authors: Jesper Grau Eriksen, Christian Maare, Jørgen Johansen , Hanne Primdahl, Åse Bratland , Claus Andrup Kristensen , Maria Andersen, Jan Alsner , Jens Overgaard
The first randomized study to investigate the addition of zalutumumab to curative radiotherapy for Head and Neck Squamous Cell Carcinomas (HNSCC) has concluded that the targeted therapy does not improve loco-regional control, disease-specific survival, or overall survival. These findings, from a large-scale Danish and Norwegian collaboration, challenge previous hypotheses regarding the benefit of Epidermal Growth Factor receptor (EGFR) inhibitors in this patient population.
Antibodies targeting the EGFR have been suggested as a means to reduce tumor failure and enhance survival rates when combined with radiotherapy in HNSCC patients. However, the comprehensive DAHANCA19 trial found no additional benefit from zalutumumab, a fully human monoclonal antibody against EGFR, when administered concurrently with (chemo-)radiotherapy and nimorazole.
Study Design and Methodology
Conducted by the Danish Head and Neck Cancer study group (DAHANCA) and Oslo University Hospital, this randomized, open-label, two-armed Phase III study enrolled 608 eligible patients with biopsy-verified HNSCC of the oral cavity, pharynx, and larynx between November 2007 and June 2012. Patients were randomized 1:1 to either a control arm or a zalutumumab arm.
Both arms received primary accelerated radiotherapy (predominantly 66–68 Gy, 2 Gy/fraction, 6 fractions/week) with concomitant daily hypoxic radiosensitization using nimorazole. Patients with Stage III-IV carcinomas additionally received weekly cisplatin (40 mg/m2). The zalutumumab arm mirrored the control arm’s treatment, plus zalutumumab at 8 mg/kg, with the first dose given a week before radiotherapy and continuing weekly throughout the treatment period.
The primary endpoint of the study was loco-regional failure, with secondary endpoints including disease-specific survival and overall survival. Analyses were performed on an intention-to-treat basis.
Key Findings: No Benefit Observed Across Endpoints
After a median follow-up of 59 months, the results showed no significant improvement with the addition of zalutumumab.
- Loco-regional failure: The 5-year loco-regional failure rate was 24% in the zalutumumab arm (66 failures) compared to 18% in the control arm (52 failures). The Hazard Ratio (HR) was 1.16 (95% CI, 0.84–1.59), indicating no reduction in failure with zalutumumab.
- Disease-specific survival: No difference was observed between the arms (HR 1.04; 95% CI, 0.73–1.50). The 60-month disease-specific survival rates were 80% for the zalutumumab arm and 81% for the control arm.
- Overall survival: Similarly, there was no statistically significant difference in overall survival (HR 1.21; 95% CI, 0.91–1.61). At 60 months, the actuarial survival rate was 65% in the zalutumumab arm and 71% in the control arm.
The study also investigated the potential influence of Human Papillomavirus (HPV)/p16 status, a known prognostic factor in oropharyngeal HNSCC. However, the effect of zalutumumab was not influenced by HPV/p16 status, meaning the drug did not show benefit in either HPV/p16 positive or negative subgroups. Subgroup analyses exploring benefit in elderly patients or by smoking status also yielded no positive effect.
Read About Results of Perioperative Chemoimmunotherapy In Resectable Stage II–IIIA NSCLC
Toxicity and Implications
While generally well-tolerated, the addition of zalutumumab did lead to increased acute toxicity, notably a significant rise in in-field skin reactions and a more universal rash, experienced by 92% of patients in the zalutumumab arm. Furthermore, patients receiving zalutumumab had a significantly lower chance of completing the full five cycles of cisplatin, suggesting that the added acute toxicity might have limited the administration of concurrent chemotherapy. No significant differences were recorded in peak late toxicity.
The findings align with other studies, such as the RTOG 0522 trial and the Concert 1 phase II study, which similarly found that adding EGFR inhibitors like cetuximab or panitumumab to chemoradiotherapy for HNSCC did not improve outcomes but increased toxicity. These results suggest that there may be an overlap in the mechanisms of action between EGFR inhibitors and other components of (chemo-)radiotherapy, negating any additional benefit.
Conclusion and Clinical Recommendations
This robust, first-of-its-kind randomized study provides definitive evidence that the addition of concomitant zalutumumab to primary (chemo-)radiotherapy and nimorazole for HNSCC does not improve loco-regional control, disease-specific, or overall survival at 60 months. This lack of benefit holds true regardless of whether patients received concurrent chemotherapy or their HPV/p16 status.
Based on the current evidence, there is no rationale for combining zalutumumab with (chemo-)radiotherapy in the primary treatment of Head and Neck Squamous Cell Carcinomas. These results will inform future treatment guidelines and help avoid unnecessary toxicity for patients.
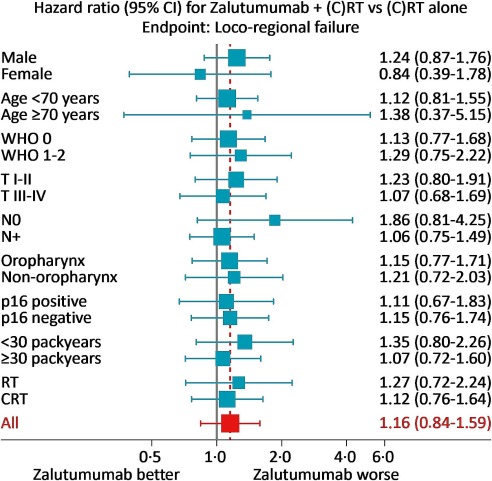
Fig. 5 Post-hoc forest-plot exploring the benefit of zalutumumab in relation to selected patient, tumour and treatment characteristics.
You Can Read Full Article on www.thegreenjounral.com
Written By Aren Karapetyan, MD


