Radiotherapy (RT) has long been a cornerstone in cancer treatment, providing local control over irradiated areas. However, recent studies have shown a phenomenon where cancer cells outside the radiation zone also regress, which is known as the abscopal effect. This effect refers to the systemic anti-tumor immune response initiated by RT, resulting in the regression of non-irradiated metastatic lesions at a distance from the primary site of radiation. Despite its intriguing potential, the precise biological mechanism behind the abscopal effect remains elusive.
Radiotherapy exerts its primary effects by directly damaging DNA in tumor cells and inducing the formation of free radicals that also harm neighboring healthy tissues. While these direct cytotoxic effects are well-understood, their influence on the immune system has garnered increased interest, particularly in relation to the abscopal effect.
The immune system’s role in the anti-tumor response triggered by RT is complex. While RT is traditionally considered immunosuppressive due to its impact on immune cells, it also has the potential to activate immune responses, especially when combined with immunotherapy.
What is the Abscopal Effect and How Can It Be Combined with Immunotherapy?
The term abscopal was coined by Mole in 1953, originating from the Latin words “ab-” (away from) and “scopus” (target), meaning “at a distance from the irradiated volume but within the same organism.” While originally used to describe the remote effects of radiation, the abscopal effect has gained new relevance in the era of immunotherapy.
The integration of immunotherapy with RT has reshaped cancer treatment strategies. Immune checkpoint inhibitors, such as those targeting CTLA-4 and PD-1, have renewed interest in the abscopal effect. These inhibitors help remove the immune system’s brakes, allowing it to attack cancer cells more effectively. When combined with RT, which can reprogram the tumor microenvironment to initiate immune responses, the abscopal effect has been seen more frequently.
RT typically works by damaging the DNA of tumor cells, but it also has a broader influence on the immune system. RT not only directly kills tumor cells but can stimulate the immune system through immunogenic cell death. This process releases cytokines and chemokines that attract immune cells like dendritic cells (DCs) to the tumor site. These cells, in turn, activate cytotoxic T lymphocytes (CTLs) that target and kill tumor cells, including those at distant metastatic sites.
However, RT’s immunosuppressive effects, such as the recruitment of regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs), can dampen the immune response. This creates a delicate balance, and strategies to enhance the abscopal effect have focused on overcoming these immune-suppressive mechanisms.
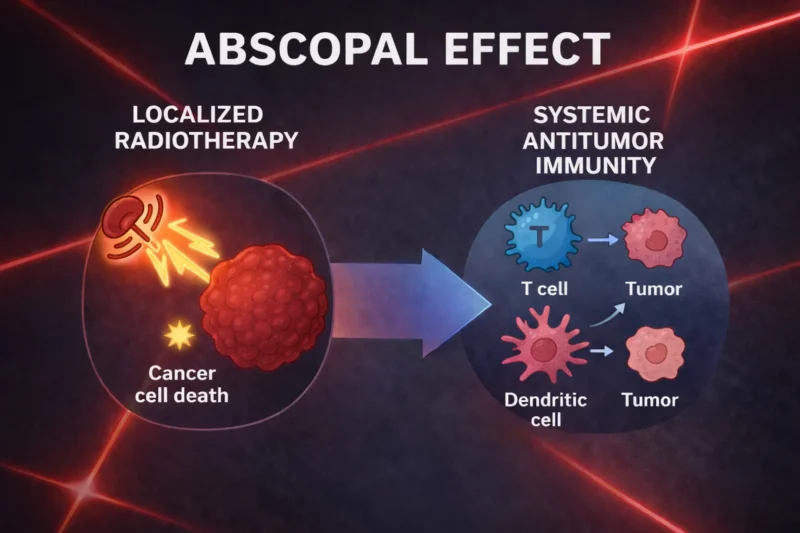
Synergy Between RT and Immunotherapy: Advancements in the Abscopal Effect
Though traditionally rare, the occurrence of the abscopal effect has been reported in several case studies, particularly in cancers considered to be immunogenic like melanoma, renal cell carcinoma, and lymphoma. These tumors respond more readily to immune stimulation, which may explain their increased susceptibility to the abscopal effect.
In an effort to enhance this rare phenomenon, researchers have tested several strategies to improve the incidence of the abscopal effect. These include the combination of RT with immune checkpoint inhibitors, cytokine therapies, dendritic cell stimulation, and even tumor vaccination. Among these, the combination of RT with immune checkpoint inhibitors such as CTLA-4 blockade has been shown to induce more frequent and robust abscopal responses.
Dewan et al. investigated the optimal dose and fractionation of RT to enhance the synergy with immunotherapy. Their research found that a regimen of 8 Gy x 3 (eight gray per fraction over three days) was most effective at inducing the abscopal effect when combined with immune checkpoint blockade. This approach appears to strike a balance between cytosolic DNA accumulation (which activates immune responses) and Trex1 activity (which could degrade the immune-activating DNA). This balance is crucial for optimizing the immune response while preventing DNA degradation that would reduce the effectiveness of RT and immunotherapy.
Read the full article: Radiotherapy’s Abscopal Effect: Mechanisms, Challenges, and Key Principles for Residents, which provides an overview of the biological basis, clinical challenges, and practical considerations of this rare but clinically relevant phenomenon.
Modern Clinical Examples and Future Directions
The use of RT in combination with immune checkpoint inhibitors has led to several promising case reports of the abscopal effect. For instance, Postow et al. were the first to demonstrate the abscopal effect in a patient with metastatic melanoma treated with both ipilimumab (a CTLA-4 inhibitor) and RT. The patient’s tumor exhibited regression at distant sites after the RT was applied to a local mass, even as the melanoma was progressing under immune therapy alone.
Since then, many more reports have surfaced, further demonstrating the promise of combining RT with immunotherapy. For example, Yazici et al. recently published a case study showing the abscopal effect in a patient with sinonasal carcinoma, where the combination of RT and pembrolizumab (a PD-1 inhibitor) resulted in the resolution of both irradiated and distant metastases.
This growing body of evidence suggests that we are entering a new era of cancer treatment, where RT and immunotherapy together may offer a powerful strategy not only for treating localized tumors but also for combating metastatic disease.
Re-irradiation Strategies: Patient Selection and Safety Considerations, Reirradiation (reRT)
Written by Nare Hovhannisyan, MD



