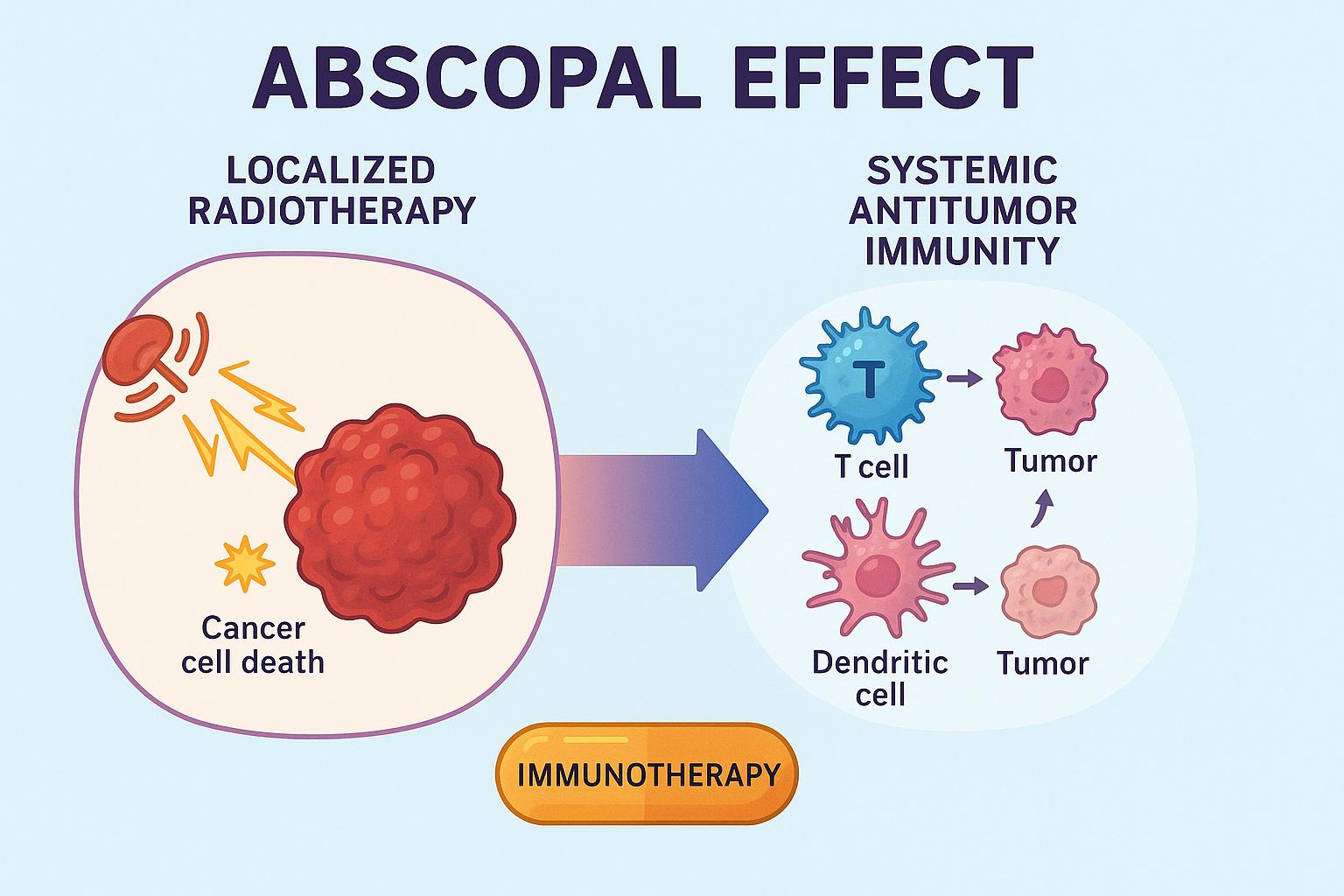
For decades, radiation therapy was viewed as a strictly localized treatment—capable of killing cancer cells within the targeted field, but incapable of producing systemic effects. Yet rare clinical observations dating back to the 1950s described something unexpected: tumors outside the radiation field shrinking after a separate lesion was irradiated. This phenomenon, termed the abscopal effect (from Latin ab — away from, and scopus — target), remained mysterious and exceedingly uncommon.
The modern immuno-oncology era has dramatically changed our understanding of the abscopal effect. What was once considered a biological curiosity is now recognized as an immune-mediated systemic phenomenon, often emerging only in the presence of immunotherapy.
This article provides a detailed scientific narrative explaining the abscopal effect, how radiotherapy triggers systemic antitumor immunity, and why immunotherapy has transformed it from a rare event into a clinically relevant therapeutic strategy.
What Is the Abscopal Effect?
The abscopal effect refers to regression of non-irradiated tumors following localized radiotherapy, mediated by the immune system. It is not a direct effect of radiation on distant tissues, but rather a systemic immune response triggered by localized tumor cell death.
Historically, the abscopal effect was extremely rare because radiation alone is insufficient to generate strong systemic immunity. Tumors have evolved multiple immunosuppressive mechanisms—including regulatory T cells, myeloid-derived suppressor cells, and checkpoint pathways—that prevent the immune system from recognizing them.
The landscape changed with the introduction of checkpoint inhibitors (anti-PD-1, anti-PD-L1, anti-CTLA-4). When these agents release inhibitory brakes on T cells, radiation-induced antigen release becomes immunologically actionable. The synergy between radiation and immunotherapy is now considered a fertile area of active research.
Mechanistic Basis: How Local Radiation Produces Systemic Immune Activation
The abscopal effect is fundamentally an immunologic cascade triggered by immunogenic cell death (ICD). Radiation produces highly immunogenic signals that transform the irradiated tumor into an in situ vaccine.
1. Immunogenic Cell Death and Tumor Antigen Release
Radiation induces:
- DNA damage
- Double-stranded breaks
- Mitochondrial stress
This results in tumor cell death accompanied by the release of tumor-associated antigens (TAAs) and danger-associated molecular patterns (DAMPs). Key DAMPs include:
- HMGB1, which stimulates dendritic cell activation
- ATP, which promotes dendritic cell recruitment
- Calreticulin exposure, serving as an “eat-me” signal
Together, these convert the irradiated tumor microenvironment into a powerful immunogenic milieu.
2. Dendritic Cell Activation and Antigen Presentation
DAMP signaling activates dendritic cells (DCs), which phagocytose dying tumor cells, process TAAs, and migrate to regional lymph nodes. There, they present antigens to naïve T cells via MHC-I and MHC-II pathways.
This results in:
- Clonal expansion of tumor-specific effector CD8+ T cells
- Differentiation of CD4+ helper T cells
- Development of memory T-cell pools
3. Systemic T-Cell Trafficking
Activated CD8+ T cells then circulate systemically. If they encounter metastatic lesions expressing similar antigens, they initiate cytotoxic killing. The outcome is tumor regression at sites far from the irradiated field.
4. Immune Checkpoints Limit the Response Without Immunotherapy
However, tumors upregulate PD-L1, secrete TGF-β, and recruit suppressive immune cells, limiting the T-cell attack. Without immunotherapy, the abscopal effect is uncommon because the activated T-cell response is rapidly shut down.
Why the Abscopal Effect Is Rare Without Immunotherapy
Without checkpoint blockade:
- antigen presentation occurs but is rapidly suppressed
- T cells become exhausted at metastatic sites
- local suppressive pathways (TGF-β, regulatory macrophages) dominate
This explains why the abscopal effect only rarely occurred before the immunotherapy era.
Clinical Evidence for the Abscopal Effect
The modern understanding of the abscopal effect—systemic tumor regression triggered by local radiotherapy—comes largely from clinical trials combining radiation with immunotherapy. While the phenomenon was historically rare, checkpoint inhibitors have made it more observable and clinically relevant.
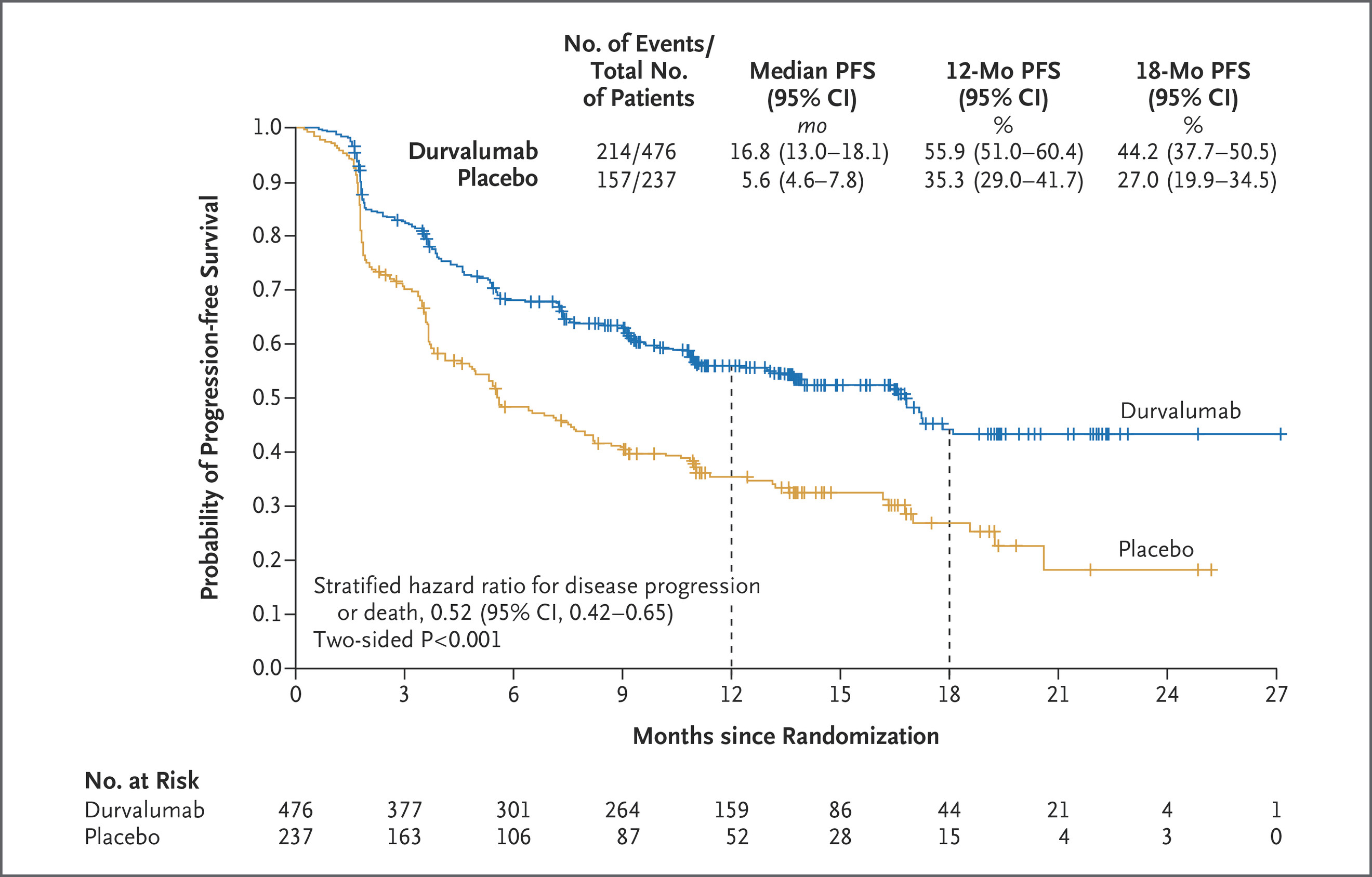
The PACIFIC trial (NCT02125461) is often cited as indirect evidence that radiotherapy can prime systemic immunity. In this phase III trial of unresectable stage III NSCLC, patients who received durvalumab after chemoradiation had dramatically improved survival compared with placebo. Median overall survival approached four years, and distant metastatic relapse was significantly reduced. Although PACIFIC did not measure the abscopal effect directly, its results suggest that radiation-induced antigen release, followed by PD-L1 blockade, enhanced systemic immune control beyond the radiation field.
Abscopal Effect
More direct evidence comes from melanoma. The first widely recognized modern abscopal case was reported by Postow et al. in 2012, when a patient on ipilimumab experienced regression of non-irradiated metastases after receiving radiotherapy to a single lesion. This observation led to prospective trials such as the Hiniker study (NCT01449279), where ipilimumab combined with stereotactic radiotherapy produced abscopal responses in roughly 25% of patients—far higher than the historically negligible rate with radiation alone. Mechanistic work from Twyman-Saint Victor further demonstrated that radiation enhances T-cell activation but also induces PD-L1 expression, explaining why combining radiation with checkpoint blockade can create a more robust and sustained systemic response.
The PEMBRO-RT trial (NCT02492568) in metastatic NSCLC provided some of the clearest prospective evidence of radiation-enhanced systemic immunotherapy. Patients who received pembrolizumab plus SBRT had double the objective response rate and nearly double the overall survival compared with pembrolizumab alone. Many responding lesions were located outside the radiation field, consistent with an abscopal-type mechanism.
Ongoing studies continue to refine how to best combine radiotherapy with immunotherapy. Trials such as TIMELY (NCT03705699) and CAIR (NCT03085719) are evaluating the optimal sequencing of radiation relative to checkpoint blockade, with early data suggesting that concurrent delivery produces stronger systemic immune activation. In melanoma, the RADVAX trial (NCT02303990) showed that adding SBRT to dual checkpoint inhibition with nivolumab and ipilimumab produced unusually high systemic response rates. Trials such as NIVES in renal cell carcinoma further support the idea that radiation can broaden the reach of immunotherapy in traditionally immunotherapy-sensitive cancers.
Together, these studies form a coherent picture: radiotherapy provides immunogenic tumor antigens and inflammatory signals, while checkpoint inhibitors maintain and amplify the resulting T-cell response. The abscopal effect remains uncommon, but in the immunotherapy era, it is increasingly supported by scientific evidence and may represent an emerging therapeutic strategy, especially when radiation and immunotherapy are thoughtfully integrated.
Immunologic Barriers: Why the Abscopal Effect Still Fails in Some Tumors
Not all tumors support an abscopal response, even with immunotherapy. Key resistance mechanisms include:
1. Immunologically “cold” tumors
Tumors with low T-cell infiltration (e.g., MSS colorectal cancer) are less likely to mount systemic immune responses.
2. TGF-β–driven immune exclusion
As demonstrated in colorectal cancer (Nature Genetics 2025 study), TGF-β creates:
- barriers to T-cell entry
- suppression of intratumoral T-cell expansion
3. M2 macrophage dominance
Suppressive macrophages produce IL-10 and TGF-β, limiting antigen presentation.
4. PD-L1 upregulation without adequate T-cell priming
Checkpoint blockade is ineffective if T cells never reach the tumor.
Understanding these barriers helps residents appreciate why combining radiation with immunotherapy is promising—but not universally successful.