Defective mismatch repair (dMMR) and microsatellite instability (MSI-H) define a biologically distinct subset of colorectal cancer characterized by high tumor mutational burden and marked sensitivity to immune checkpoint inhibition. While adjuvant chemotherapy remains standard for stage II–III disease, dMMR/MSI tumors derive limited benefit from fluoropyrimidine-based regimens, raising concerns about overtreatment and unnecessary toxicity.
In recent years, PD-1 inhibitors have transformed outcomes in metastatic dMMR/MSI colorectal cancer and have begun to move into earlier disease settings. Neoadjuvant immunotherapy is particularly compelling, offering the possibility of deep pathologic responses, de-escalation of adjuvant therapy, and, in selected cases, avoidance of radical surgery—an especially meaningful consideration for rectal cancer.
The study, titled “Neoadjuvant Immunotherapy With Prolgolimab in Patients With Locally Advanced Microsatellite Instability/Defective Mismatch Repair Colorectal Cancer,” was published as an Original Report in JCO Precision Oncology on December 10, 2025.
The investigation was led by Olesya Kuznetsova, MD, with co-authors Albina Zagidullina, MD; Natalia Drobot, MD, PhD; Aleksander Prokopiev, MD; Maxim Ivanov, PhD; Mikhail Fedyanin, MD, PhD; Zaman Mamedli, MD, PhD; Vyacheslav Aliev, MD, PhD; Andrey Polynovsky, MD, PhD; Khasan Dzhumabaev, MD, PhD; Aleksander Aniskin, MD, PhD; Anna Stroganova, MD, PhD; Ivan Karasev, MD, PhD; and Alexey Tryakin, MD, PhD.
What is Prolgolimab and How Does it Work?
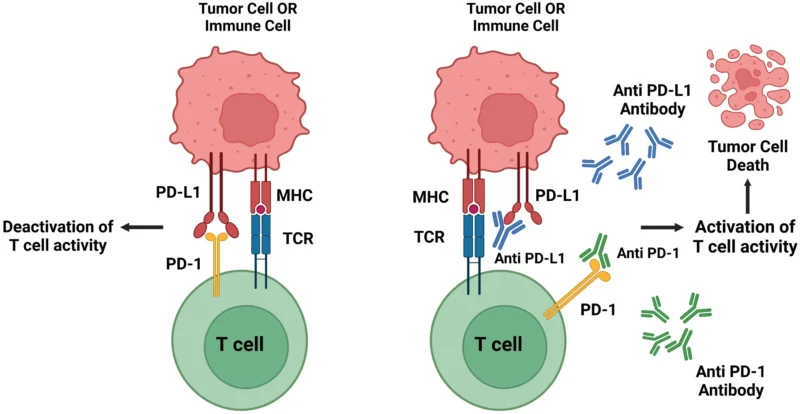
Prolgolimab (formerly BCD-100) is a monoclonal antibody designed to enhance the body’s immune response against cancer. It belongs to the class of immune checkpoint inhibitors and specifically targets the programmed cell death-1 (PD-1) receptor, a key regulatory molecule expressed on activated T lymphocytes.
Under normal conditions, the PD-1 pathway acts as a physiological brake on the immune system, preventing excessive immune activation and autoimmunity. Many cancers—including dMMR/MSI-high colorectal tumors—exploit this pathway by expressing PD-L1 and PD-L2 within the tumor microenvironment, thereby engaging PD-1 on activated T cells and suppressing antitumor immune activity. By binding to PD-1, prolgolimab blocks this inhibitory signal, allowing cytotoxic T cells to remain active, recognize tumor neoantigens, and destroy malignant cells.
A distinguishing feature of prolgolimab is its Fc-silenced IgG1 backbone, achieved through the LALA mutation in the Fc region of the antibody. This modification prevents interaction with Fc-gamma receptors on immune cells, thereby minimizing antibody-dependent effector functions such as ADCC or complement activation. Functionally, this design is intended to preserve immune checkpoint blockade while minimizing Fc-mediated effector functions, including antibody-dependent cellular cytotoxicity and complement activation.
source: Frontiers
Methods and Endpoints
This prospective, open-label, nonrandomized phase II trial enrolled adults with stage II–III colorectal adenocarcinoma confirmed to be dMMR/MSI by PCR and immunohistochemistry. Patients received prolgolimab at 1 mg/kg intravenously every two weeks, with a planned treatment duration of six months prior to surgical resection. In cases of surgery refusal, immunotherapy could be continued for up to one year, at the discretion of the treating team and patient.
The primary endpoint was a composite of pathologic complete response (pCR) in operated patients and clinical complete response (cCR) in nonoperated patients, reflecting both surgical and nonoperative outcomes. Secondary endpoints included pCR, major pathologic response (MPR), objective response rate, safety, disease-free survival, and overall survival.
Results
Thirty patients were enrolled, most of whom had high-risk disease features, including stage III tumors and nodal involvement. Right-sided colon cancers predominated, and half of the cohort harbored BRAF V600E mutations, underscoring the aggressive biology of this population.
Following neoadjuvant therapy, 26 patients underwent surgery, and the study met its primary endpoint. Key efficacy outcomes included:
- pCR + cCR achieved in 56.7% of all enrolled patients
- pCR observed in 61.6% of operated patients
- MPR achieved in 80.8% of resected tumors
- Radiographic objective response rate of 89.7%
Three patients with low rectal primary tumors refused surgery—one after achieving a clinical complete response and two despite partial clinical responses—highlighting the potential for organ preservation in carefully selected cases.
Radiographic tumor shrinkage was substantial in most patients, but imaging response did not reliably predict pathologic outcomes. Several patients with residual radiographic abnormalities were found to have complete tumor eradication at surgery, and pseudoprogression was observed in a minority of cases, emphasizing the limitations of conventional imaging after immunotherapy.
At a median follow-up of 19 months, disease control remained durable:
- 18-month disease-free survival: 90%
- 18-month overall survival: 90%
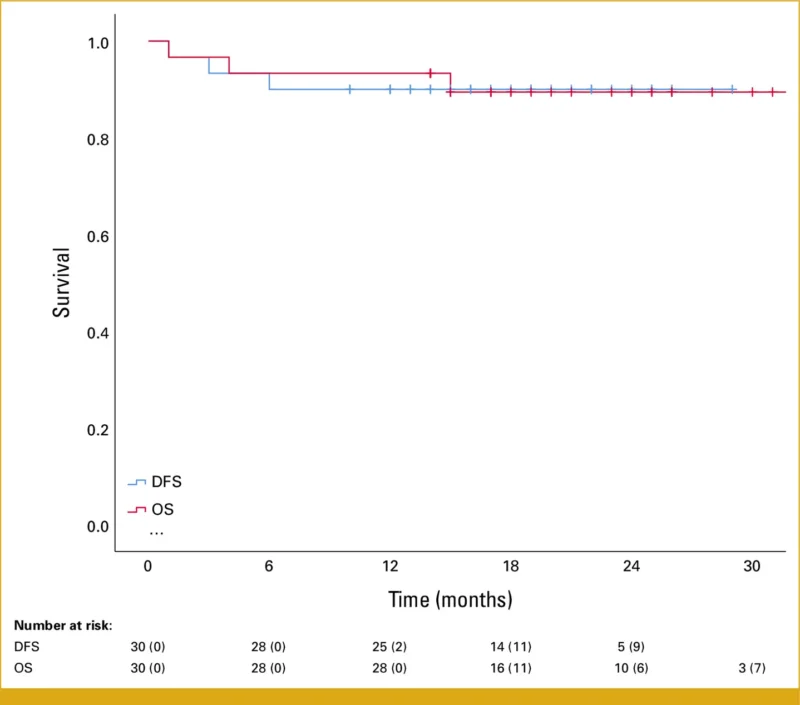
Only two confirmed disease progressions were observed. Three deaths occurred during follow-up, none of which were attributed to prolgolimab treatment.
Prolgolimab was generally well tolerated. Immune-related adverse events were mostly low grade, with only one patient (3.3%) experiencing a grade 3–4 toxicity, which resolved with corticosteroid therapy.
Conclusion
This phase II study demonstrates that neoadjuvant prolgolimab induces deep and durable responses in locally advanced dMMR/MSI colorectal cancer, with pCR rates comparable to other PD-1–based strategies and a favorable safety profile. Beyond response magnitude, the findings highlight a broader shift in treatment goals—from simply improving survival to reducing treatment intensity and preserving organ function where safely possible.
At the same time, the results underscore critical challenges that must be addressed before nonoperative management can be widely adopted, including the poor correlation between imaging and pathologic response and the need for integrated assessment using endoscopy, pathology, and molecular biomarkers. As neoadjuvant immunotherapy continues to redefine standards of care in dMMR/MSI colorectal cancer, studies such as this help clarify not only how well these therapies work, but how much treatment patients may ultimately be able to avoid.
Full article is available on JCO Precision Oncology.


