Pancreatic ductal adenocarcinoma (PDAC) remains one of the most lethal cancers, with surgery offering the only chance for cure. However, outcomes after surgery alone are poor, and the role of neoadjuvant therapy in improving survival continues to be an important question. The PREOPANC-2 trial was designed to compare two strategies: neoadjuvant FOLFIRINOX versus gemcitabine-based chemoradiotherapy (CRT) followed by adjuvant gemcitabine in patients with resectable or borderline resectable disease.
PREOPANC-2 Trial Design
PREOPANC-2 was a nationwide, investigator-initiated, open-label, phase 3 randomized trial across 19 hospitals in the Netherlands. Patients with resectable or borderline resectable PDAC and WHO performance status 0–1 were randomized 1:1 to FFX or CRT using a minimisation technique, stratified by resectability and center.
- FOLFIRINOX (FFX) Group: Patients received eight cycles of full-dose neoadjuvant FOLFIRINOX every 14 days, followed by surgery without adjuvant therapy. Dose adjustments were allowed for toxicity, and granulocyte colony-stimulating factor (G-CSF) prophylaxis was recommended. Restaging CT scans were performed after four cycles. Surgery was scheduled 3–6 weeks after the final chemotherapy cycle. The FFX group had a planned total treatment duration of 22 weeks.
- Chemoradiotherapy (CRT) Group: Patients received three cycles of neoadjuvant gemcitabine, with hypofractionated radiotherapy (36 Gy in 15 fractions) during the second cycle, followed by surgery and four cycles of adjuvant gemcitabine. Dose modifications were allowed based on toxicity, with restaging CT scans before surgery. For the CRT group, the total treatment duration was set at 40 weeks.
PREOPANC-2 Trial Endpoints
The primary endpoint was overall survival (OS). Secondary endpoints included progression-free survival (PFS), resection and R0 resection rates, pathological response, treatment feasibility, and grade ≥3 adverse events. Tumor marker response, postoperative complications, and locoregional or distant recurrence were also evaluated.
Results of PREOPANC-2
The results of the PREOPANC-2 trial were published in The Lancet Oncology, September 2025. Between June 5, 2018, and January 28, 2021, 375 patients were randomly assigned to receive either neoadjuvant FOLFIRINOX (FFX, n=188) or gemcitabine-based chemoradiotherapy (CRT, n=187). Six patients were excluded due to ineligibility or immediate withdrawal of consent, leaving a modified intention-to-treat population of 369 patients (FFX n=185; CRT n=184). The cohort included 208 men (56%) and 161 women (44%), with baseline characteristics well balanced between groups.
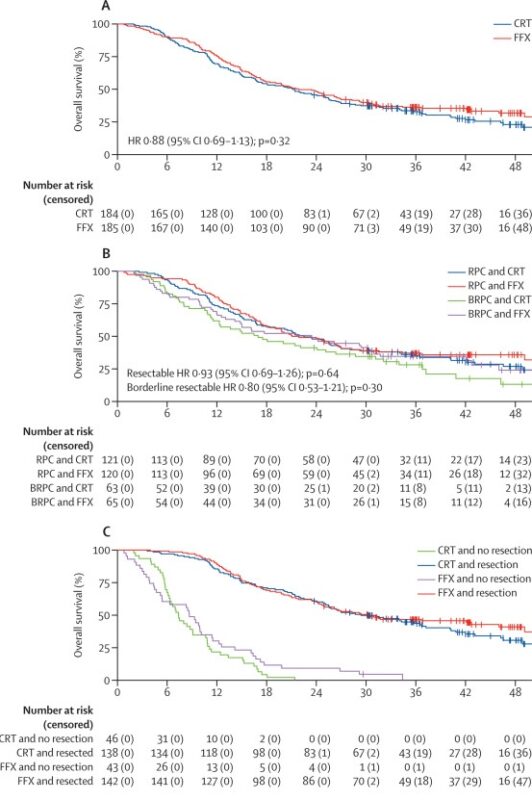
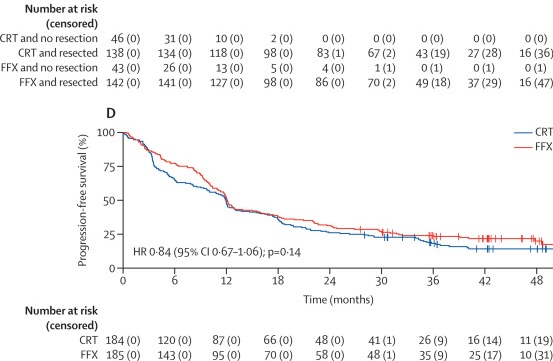
After a median follow-up of 42.3 months, median overall survival was 21.9 months (95% CI 17.7–27.0) in the FFX group versus 21.3 months (16.8–25.5) in the CRT group (HR 0.88; 95% CI 0.69–1.13; p=0.32). One- and three-year survival rates were 76% and 36% for FFX, compared with 70% and 33% for CRT. Prespecified subgroup analyses showed no significant heterogeneity of treatment effect, with similar outcomes in resectable and borderline resectable PDAC. Progression-free survival was also comparable between groups (12.1 months vs 11.9 months, HR 0.84, p=0.14).
Adverse Events
The most common grade 3–4 adverse events were neutropenia (43 [25%] of 175 in the FFX group vs 38 [22%] of 176 in the CRT group), diarrhea (41 [23%] vs 2 [1%]), and leukopenia (14 [8%] vs 26 [15%]). Serious adverse events occurred in 85 (49%) patients in the FFX group compared with 75 (43%) in the CRT group (p=0·26). Overall, adverse events of grade 3 or worse occurred in 117 (67%) patients in the FFX group versus 106 (60%) in the CRT group (p=0·20). Treatment-related deaths were rare, occurring in two (1%) patients in the FFX group (multi-organ failure and intestinal mucositis) and one (1%) patient in the CRT group (upper gastrointestinal hemorrhage).
Conclusion
In the PREOPANC-2 trial, neoadjuvant FOLFIRINOX did not demonstrate superior overall survival compared with neoadjuvant gemcitabine-based chemoradiotherapy followed by adjuvant gemcitabine in patients with resectable or borderline resectable pancreatic ductal adenocarcinoma. Secondary outcomes—including progression-free survival, resection rates, R0 resection, and pathological complete response—were largely similar, with the CRT group showing a higher rate of ypN0 resections. Both regimens exhibited manageable toxicity, though FOLFIRINOX was associated with a higher incidence of multiple grade ≥3 adverse events.
These results support the use of either neoadjuvant strategy, with treatment selection guided by patient performance status, comorbidities, and center experience. Future studies should focus on predictive biomarkers and clarify the optimal sequencing and duration of neoadjuvant therapy to further personalize treatment in this population.

You can read more about ESMO GI 2025 Highlights: BXCL701 + Pembrolizumab in Second-Line Advanced PDAC on OncoDaily.