In a Nature Medicine report from the phase 2 PRADO trial (OpACIN-neo cohort), investigators presented the first 5-year survival outcomes and an updated biomarker analysis for neoadjuvant ipilimumab plus nivolumab in patients with stage III macroscopic melanoma. In PRADO, 99 patients received neoadjuvant ipilimumab (1 mg/kg) plus nivolumab (3 mg/kg), followed by pathologic response–guided surgical and adjuvant strategies, with the index lymph node (ILN) used as the central site for response assessment.
The update arrives at a clinically important moment: neoadjuvant ipilimumab plus nivolumab has moved into standard therapy for stage III melanoma based on the phase 3 NADINA trial, yet durable long-term outcomes and practical baseline biomarkers for treatment personalization have remained limited.
Five-Year Survival Outcomes: Durable Disease Control Signals
With a data cutoff of January 6, 2025 and a median follow-up of 60 months, PRADO reported strong long-term survival endpoints:
- 5-year event-free survival (EFS): 71%
- 5-year relapse-free survival (RFS): 74%
- 5-year distant metastasis–free survival (DMFS): 79%
- 5-year overall survival (OS): 86%
- Exploratory melanoma-specific survival (MSS): 88%
Median EFS, RFS, DMFS, OS, and MSS were not reached at the time of analysis.
Long-Term Immune Toxicity: Chronic Low-Grade irAEs Are Common
The long-term safety profile was characterized by persistent, mostly low-grade immune-related adverse events:
- Grade ≥3 irAEs occurred in 30% of the total PRADO population.
- Among patients alive at cutoff, ongoing grade 1–2 irAEs were present in 69%, predominantly vitiligo and hypothyroidism.
- The report noted no ongoing grade 3–4 irAEs at 5 years, but emphasized that chronic endocrinopathies can remain clinically meaningful due to lifelong hormone replacement requirements in subsets of patients.
Pathologic Response as the Central Prognostic Signal
Patients were grouped by International Neoadjuvant Melanoma Consortium criteria, and PRADO reinforced the consistent observation across neoadjuvant melanoma programs: major pathologic response (MPR) remains one of the strongest correlates of long-term outcome.
The report highlighted that outcomes in PRADO were particularly favorable in patients achieving MPR (≤10% residual viable tumor), and it also noted that survival appeared similar between pCR (0%) and near-pCR (>0% to ≤10%) in PRADO.
A notable discussion point in the manuscript is the unexpectedly poor outcome in PRADO patients with pathologic partial response (pPR; >10% to ≤50%), contrasted with more favorable pPR outcomes historically observed in other cohorts such as OpACIN-neo. The authors explored potential explanations, including baseline characteristic differences (including a higher proportion of low TMB in PRADO pPR), and the possibility of response misclassification when ILN response is used without full TLND in all patients.
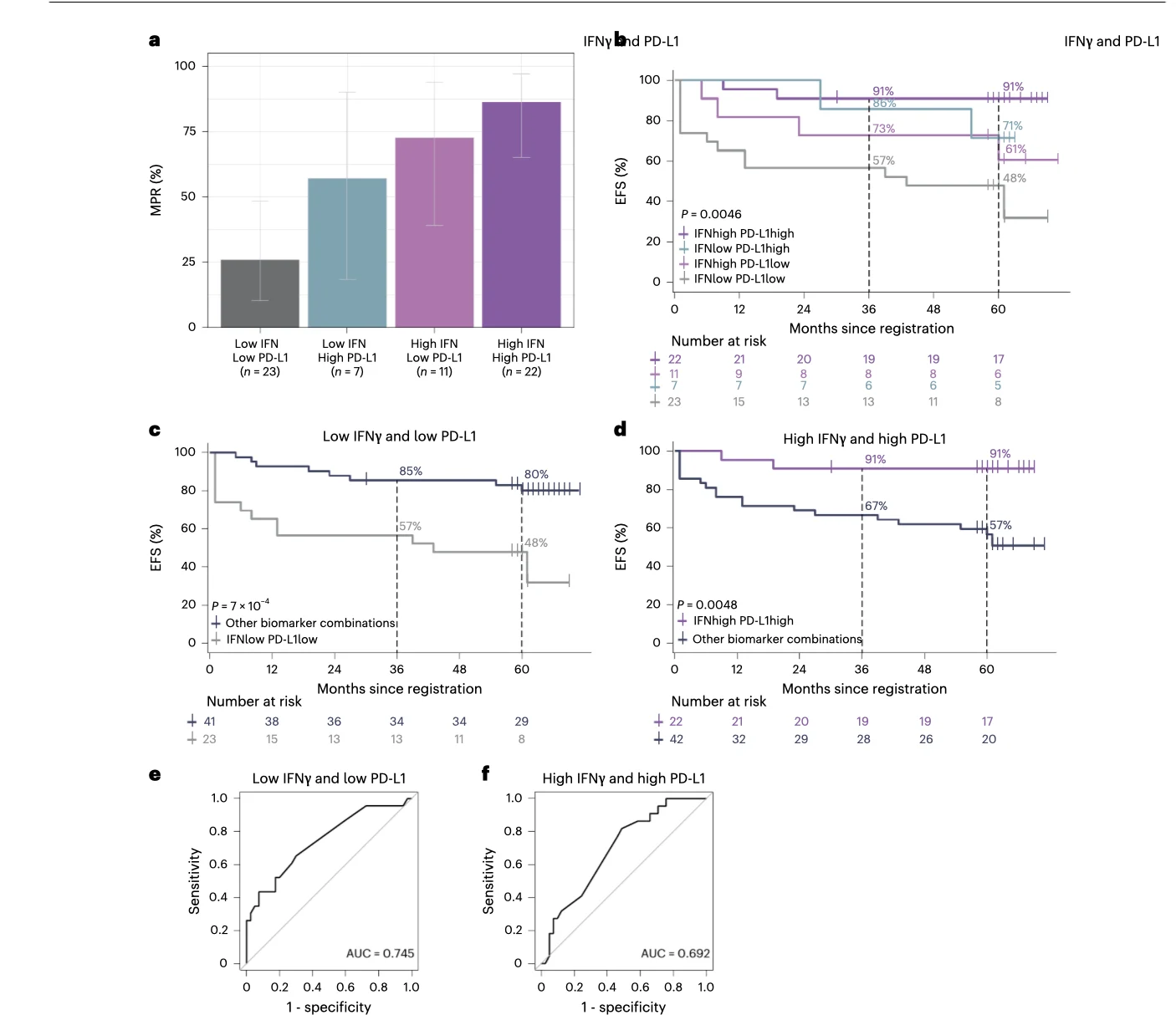
Baseline Biomarkers: IFNγ and PD-L1 Emerge as Practical Signals
The PRADO biomarker analysis focused on three baseline parameters: tumor mutational burden (TMB), interferon-gamma (IFNγ) signature, and PD-L1 tumor proportion score (TPS).
IFNγ signature
- Patients with high IFNγ had higher MPR rates (reported as 77% vs 34% in low IFNγ).
- High IFNγ was associated with favorable EFS (5-year rate 81% vs 54%, statistically significant after multiplicity adjustment).
PD-L1 TPS ≥1%
- PD-L1 expression ≥1% was associated with higher MPR rates (reported as 79% vs 47% for PD-L1 <1%).
- PD-L1 ≥1% was associated with improved EFS (5-year rate 88% vs 60%, significant after multiplicity adjustment).
TMB
- High TMB correlated with higher MPR rate (78% vs 35% for low TMB), but in PRADO TMB was not consistently associated with long-term survival endpoints after adjustment—an important contrast to some prior datasets and to OpACIN-neo, where survival differences by TMB appeared more pronounced.
PRADO tria
The “Triple Biomarker” Model: Identifying Extremes of Benefit
One of the most clinically striking outputs in this PRADO update was the combined baseline model using all three biomarkers:
High TMB + high IFNγ + PD-L1 ≥1% (“triple high”)
- 100% MPR
- 100% 5-year EFS
Low TMB + low IFNγ + PD-L1 <1% (“triple low”)
- 18% MPR
- 41% 5-year EFS
The study also examined a simplified, more feasible doublet approach (IFNγ + PD-L1), noting that it produced only slightly lower discrimination than the full triple model, which matters because clinical-grade TMB can be time- and resource-intensive.
Clinical Meaning: Response-Adapted Surgery, Biomarker-Guided Personalization
PRADO was positioned as a landmark trial because it prospectively tested surgical de-escalation, using the ILN as a representative site for pathologic response assessment. The long-term update supports the durability of outcomes—especially in MPR responders—and frames baseline biomarkers (particularly IFNγ and PD-L1) as tools that could support:
- de-escalation (avoiding unnecessary toxicity or surgery in likely responders),
- escalation (identifying likely non-responders for intensified strategies), and
- smarter selection for alternative neoadjuvant regimens (for example, PD-1 monotherapy in selected patients to reduce chronic toxicity).
Conclusion
The 5-year PRADO update provides long-term survival benchmarks for neoadjuvant ipilimumab plus nivolumab in stage III macroscopic melanoma and reinforces two clinically defining themes: MPR remains a robust surrogate marker for long-term outcome, and baseline immune biology—especially IFNγ signature and PD-L1 expression—appears to meaningfully stratify benefit.
The “triple high” baseline biomarker subgroup achieving 100% MPR and 100% 5-year EFS, contrasted with the “triple low” subgroup showing 18% MPR and 41% EFS, illustrates the direction the field is moving toward: response-adapted, biomarker-guided personalization of neoadjuvant immunotherapy intensity, surgery, and adjuvant management.
You Can Read All Article Here


