Presented at ASCO 2025 by Dr. Reena Engineer from Tata Memorial Centre, POLCAGB Phase III randomized trial (NCT02867865) explored the role of neoadjuvant chemoradiotherapy (NACRT) versus neoadjuvant chemotherapy (NACT) in patients with locally advanced gallbladder cancer (LAGBC). This is one of the first randomized trials in this rare and aggressive cancer, designed to assess whether adding radiation to chemotherapy could enhance resection rates and overall survival.
Eligible patients had T3/T4 gallbladder adenocarcinoma with liver infiltration (<5 cm), N1 disease, limited vascular involvement, or biliary/duodenal/colonic abutment, and were deemed initially unsuitable for upfront curative surgery.
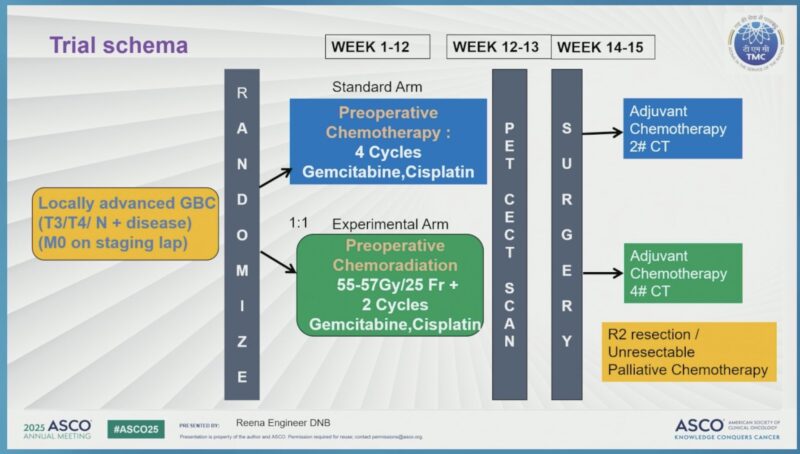
POLCAGB Trial Design and Objectives
Patients were randomized 1:1 to either:
- NACT Arm: 4 cycles of gemcitabine plus platinum
- NACRT Arm: Concurrent chemoradiation (55–57 Gy + gemcitabine), followed by 2 cycles of chemotherapy
Following neoadjuvant therapy, patients were reassessed for surgical resection. The primary endpoint of the trial was median overall survival (OS), with secondary endpoints including event-free survival (EFS), R0 resection rate (complete tumor removal), and the rate of postoperative complications.
Although the original target enrollment was 314 patients, slow recruitment led to a planned interim analysis after 124 patients were enrolled across two centers. This interim data provided valuable insights into the comparative benefits of NACRT versus chemotherapy alone in improving surgical outcomes and survival in LAGBC.
Results of POLCAGB Trial
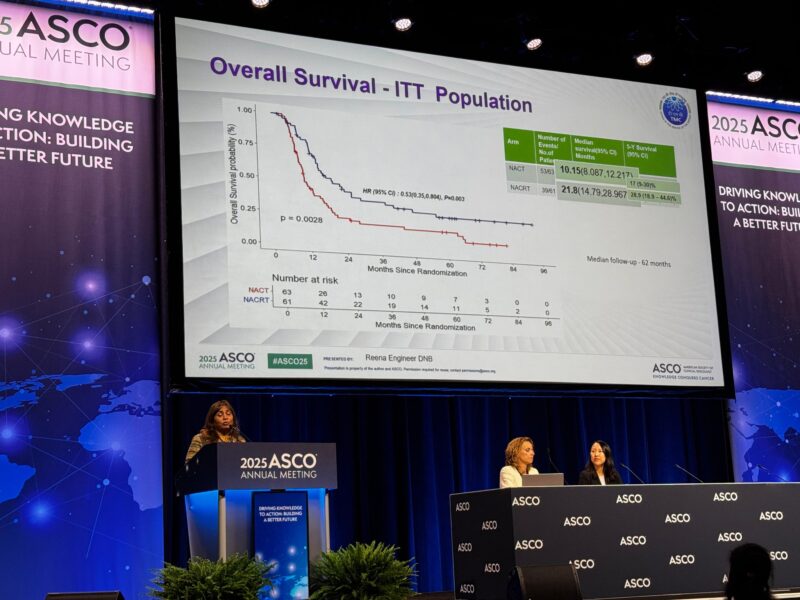
Between October 2016 and September 2024, 124 patients with locally advanced gallbladder cancer were enrolled and randomized to receive either neoadjuvant chemotherapy (NACT) or neoadjuvant chemoradiotherapy (NACRT). At the time of analysis, 93 overall survival events had occurred, with a median follow-up of 62 months (range 6.9 to 94 months).
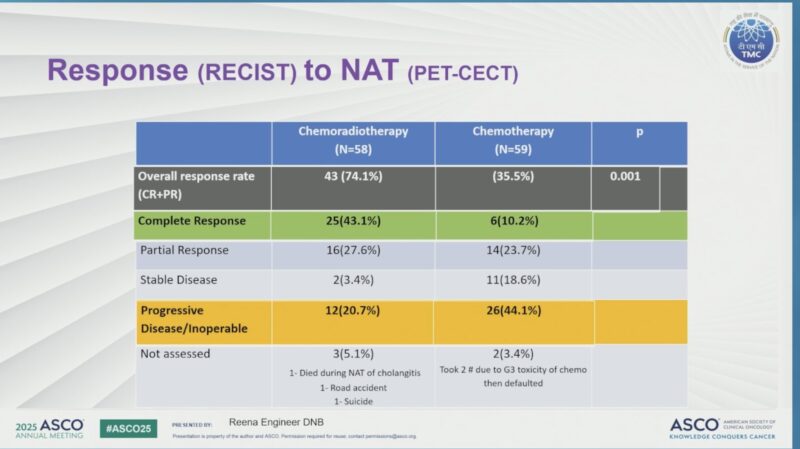
A higher proportion of patients in the NACRT arm underwent surgical exploration compared to the NACT group (65% vs. 45.3%, p=0.03). This translated into a significantly higher rate of complete tumor removal (R0 resection) in the NACRT arm, with 51.6% of patients achieving R0 resection versus 29.7% in the NACT arm (p=0.01).
Survival outcomes also favored the NACRT group:
- Median overall survival (OS) was 21.8 months (95% CI: 14.6–29.14) compared to 10.1 months (95% CI: 8.5–11.7) in the chemotherapy arm (hazard ratio 0.56, 95% CI: 0.37–0.84, p=0.006).
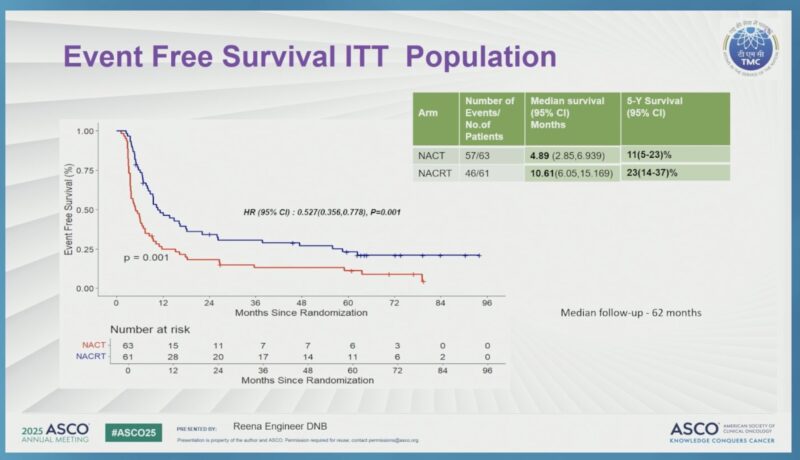
- Event-free survival (EFS) was 10.6 months (95% CI: 6.07–15.5) with NACRT, versus 4.89 months (95% CI: 3.06–6.73) with NACT (hazard ratio 0.58, 95% CI: 0.39–0.85, p=0.006).
- The estimated 5-year survival rate was higher in the NACRT group at 27% (95% CI: 17.7–43%) compared to 18% (95% CI: 10–31%) in the NACT group.
Safety and Tolerability
Surgical exploration rates were higher in the NACRT arm (65%) compared to NACT (45.3%, p=0.03).
Postoperative complications (Clavien-Dindo grade ≥3) were seen in:
- NACT: 18.18%
- NACRT: 28.12% (p=0.30)
While there was a trend toward higher surgical morbidity with NACRT, this did not reach statistical significance.
Read Full Abstract on ASCO 2025 Website
What People Are Saying About POLCAGB?
Dr. Arndt Vogel, a clinician-scientist specializing in liver cancer and precision oncology at Toronto General Hospital/Princess Margaret Cancer Center and MHH, shared insights from the POLCAGB Phase 3 trial on his X page, stating:
“Perioperative radiochemotherapy vs Gem/Cis in locally advanced gallbladder cancers ASCO25
POLCAGB phs-III
ORR 74% vs 35%
mEFS 10 vs 4.8 mo
mOS 21.8 vs 10 mo
small trial, but very interesting efficacy data in cT3/4 cN+”
Key Takeaways
The study demonstrated that neoadjuvant chemoradiation (NACRT) significantly improved outcomes in patients with locally advanced gallbladder cancer (LAGBC). Patients who received NACRT had notably higher rates of complete tumor removal and were more likely to undergo surgery compared to those receiving chemotherapy alone.
Most importantly, median overall survival more than doubled with NACRT, underscoring its potential to dramatically impact patient prognosis. The treatment was also generally well tolerated, with manageable postoperative complications. These results offer compelling evidence to support a shift in the standard treatment approach for LAGBC, favoring the integration of NACRT in this setting.
More posts featuring ASCO25.


