At the EHA 2025 Congress, Dr. Matthew Matasar, MD, presented the highly anticipated results of the Phase III POLARGO trial during a Plenary Session, marking a pivotal moment in the management of relapsed/refractory diffuse large B-cell lymphoma (R/R DLBCL).
The study evaluated the addition of polatuzumab vedotin to the R-GemOx regimen (rituximab, gemcitabine, and oxaliplatin) in patients ineligible for autologous stem cell transplant. With limited treatment options for this high-risk population, the findings revealed a statistically significant improvement in overall survival (OS) with the Pola-R-GemOx combination, offering a new therapeutic pathway for patients not previously treated with polatuzumab vedotin.
Background
Polatuzumab vedotin is approved for use in both frontline and relapsed/refractory (R/R) diffuse large B-cell lymphoma (DLBCL), with the latter indication involving its combination with bendamustine and rituximab (Tilly H, et al. 2022; Sehn L, et al. 2019). Despite available therapies, alternative treatment options are still needed for patients with R/R DLBCL.
The POLARGO trial (NCT04182204) is a global, randomized, Phase III study designed to evaluate the efficacy and safety of polatuzumab vedotin in combination with rituximab, gemcitabine, and oxaliplatin (Pola-R-GemOx) versus rituximab, gemcitabine, and oxaliplatin (R-GemOx) in patients with R/R DLBCL after at least one prior line of therapy who are ineligible for autologous stem cell transplant.
Methods
Patients with histologically confirmed R/R DLBCL, including transformed indolent lymphoma, and ineligible for ASCT were enrolled after ≥1 prior lines of therapy. After a safety run-in phase (n=15), 255 patients were randomized 1:1 to:
- Pola-R-GemOx: polatuzumab vedotin (1.8 mg/kg) + rituximab (375 mg/m²), gemcitabine (1000 mg/m²), and oxaliplatin (100 mg/m²)
- R-GemOx alone (same doses)
Treatment was administered every 21 days for up to 8 cycles.
Primary endpoint: Overall survival (OS)
Secondary endpoints: Progression-free survival (PFS), objective response rate (ORR), complete response rate (CRR), and safety
Study Design
After a safety run-in phase that included 15 patients, a total of 255 patients were randomized in a 1:1 ratio to receive either polatuzumab vedotin in combination with rituximab, gemcitabine, and oxaliplatin, or R-GemOx alone. Treatment was administered every 21 days for up to eight cycles. Eligible patients included those with DLBCL not otherwise specified or transformed indolent lymphoma, who had received at least one prior line of therapy.
The primary endpoint was overall survival, with secondary endpoints including progression-free survival, objective response rate, and complete response rate assessed by PET-CT. Safety assessments included the incidence and severity of adverse events based on NCI CTCAE v5.0.
- Design: Global, randomized, open-label Phase III trial
- Population: 270 enrolled; 255 randomized
- Median age: 66 years (range: 20–89)
- 65.2% had only 1 prior line of therapy
- 58.1% had primary refractory disease
- Median OS follow-up: 24.6 months
- Gene expression profiling included activated B-cell (ABC) and germinal center B-cell (GCB) subtypes
Results
Pola-R-GemOx demonstrated a significant survival benefit over R-GemOx in transplant-ineligible patients with R/R DLBCL. Despite increased rates of peripheral neuropathy and infections, the treatment remained tolerable and effective, offering a valuable alternative that avoids T-cell depletion. These findings support the incorporation of polatuzumab vedotin into GemOx regimens for selected patients, expanding options beyond traditional salvage therapie
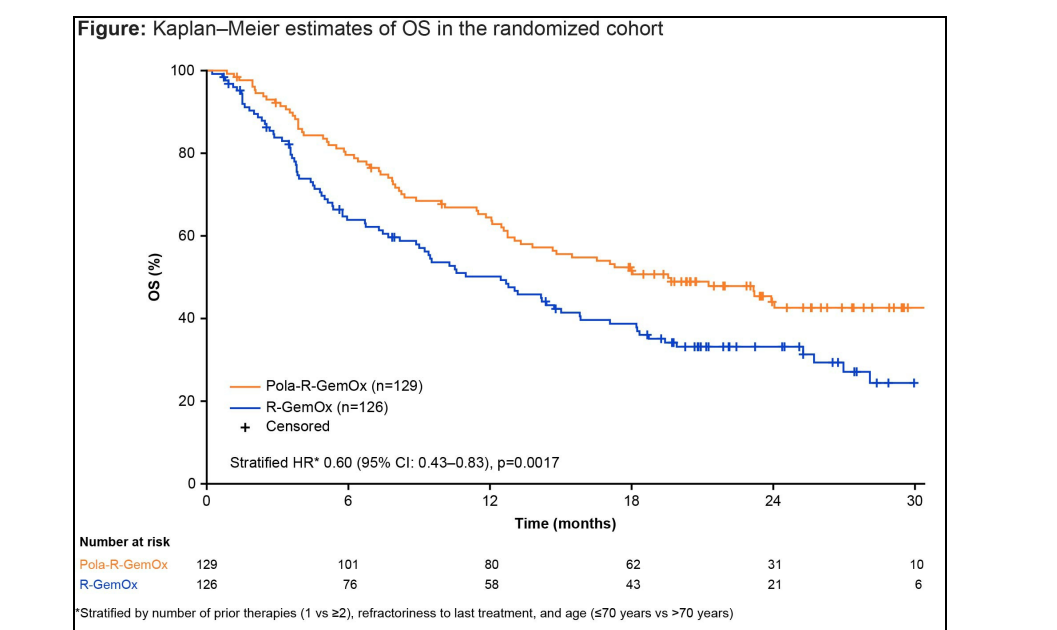
Median OS:
- Pola-R-GemOx: 19.5 months (95% CI: 13.3–NE)
- R-GemOx: 12.5 months (95% CI: 8.9–15.8)
- Hazard Ratio (HR): 0.60 (95% CI: 0.43–0.83), p=0.0017
- 40% reduction in risk of death with Pola-R-GemOx
Subgroup Benefit
- ABC subtype: HR 0.53 (95% CI: 0.3–0.9)
- GCB subtype: HR 0.54 (95% CI: 0.3–0.9)
Secondary Endpoints
- PFS and response rates (ORR, CRR) favored Pola-R-GemOx
- Full data pending in supplementary materials
Safety Grade 3–4
- AEsPola-R-GemOx: 57.0%
- R-GemOx: 58.4%
- Peripheral neuropathy (any grade)
- Pola-R-GemOx: 57.0% (mostly Grade 1)
- R-GemOx: 28.8%
- Grade 3 PN: 5 patients in Pola-R-GemOx arm
Key Takeaway Messages
- The POLARGO trial met its primary and all key secondary endpoints
- Pola-R-GemOx resulted in a statistically significant and clinically meaningful improvement in OS
- Risk of death reduced by 40% compared to R-GemOx
- Toxicity profile consistent with known components; PN and infections were more common but manageable
- Offers a new, transplant-sparing option for R/R DLBCL patients not previously treated with polatuzumab vedotin
You can read the full abstract here


