The PLANeT trial was designed to evaluate whether a flat, low dose of pembrolizumab (50 mg every 6 weeks) could preserve the efficacy of immunotherapy when combined with standard dose-dense neoadjuvant chemotherapy in patients with stage II–III triple-negative breast cancer (TNBC). This approach aimed to substantially reduce treatment costs and improve access to immune checkpoint inhibition, particularly in low- and middle-income settings.
Neoadjuvant chemotherapy followed by surgery represents the established standard of care for localized TNBC. The addition of pembrolizumab to neoadjuvant chemotherapy has previously demonstrated meaningful improvements in pathological complete response (pCR) and event-free survival, most notably in the KEYNOTE-522 trial. However, widespread adoption of standard-dose immunotherapy remains constrained by cost, infrastructure requirements, and limited availability, creating a critical need for effective, resource-adapted treatment strategies.

New Paper Alert: Immunotherapy Success in TNBC: Key Trials and Current Standards of Care
Study Design
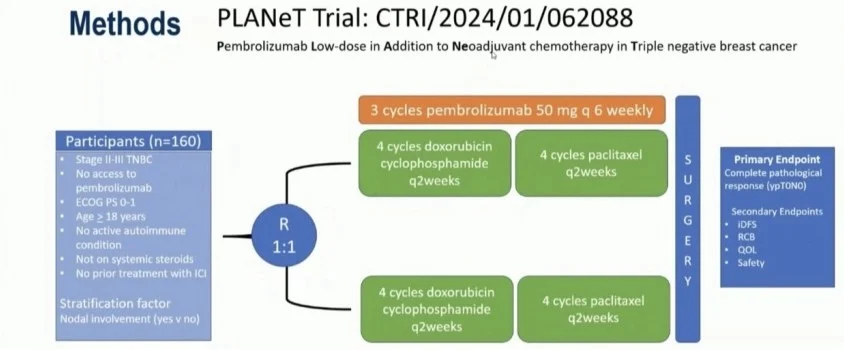
PLANeT was a phase II, randomized, open-label, investigator-initiated trial conducted at AIIMS New Delhi and affiliated cancer centers.
- Population: Previously untreated stage II–III TNBC
- Randomization: 1:1
Arms:
- Dose-dense neoadjuvant chemotherapy (AC → paclitaxel)
- Same chemotherapy plus low-dose pembrolizumab (50 mg every 6 weeks × 3 cycles)
- Primary endpoint: Pathological complete response (ypT0N0)
- Key secondary endpoints: Invasive disease-free survival, residual cancer burden, safety, quality of life
Patients enrolled lacked access to standard-dose pembrolizumab, reflecting a real-world resource-limited population.
Patient Characteristics
Between February 2024 and February 2025, 157 patients were included in the modified intention-to-treat population.
- Median age: 45 years (IQR 38–52)
- Baseline clinical and pathological features were well balanced between treatment arms
- Surgery was completed in 152 patients
Efficacy Results
The addition of low-dose pembrolizumab resulted in a statistically significant improvement in pCR rates.
- Intention-to-treat population:
- Pembrolizumab + NACT: 53.8% pCR
- NACT alone: 40.5% pCR
- Absolute difference: 13.3%
- One-sided p value: 0.047
Patients who underwent surgery:
- Pembrolizumab + NACT: 56.7% pCR
- NACT alone: 41.0% pCR
- Absolute difference: 15.7%
- One-sided p value: 0.031
Importantly, the improvement in pCR was consistent across all prespecified subgroups, with no significant interaction effects, suggesting broad applicability rather than benefit confined to select biological subsets.
The magnitude of benefit was numerically comparable to that observed with standard-dose pembrolizumab in large phase III trials.
Safety and Tolerability
Low-dose pembrolizumab did not increase overall toxicity.
- Grade ≥3 adverse events:
- Pembrolizumab + chemotherapy: 50%
- Chemotherapy alone: 59.5%
The most common toxicities—anemia, neutropenia, neuropathy, and gastrointestinal events—were consistent with expected chemotherapy-related effects. There was no deterioration in quality of life, and immune-related toxicities were manageable.
Clinical and Global Oncology Implications
PLANeT is the first randomized trial to demonstrate that a flat low dose of pembrolizumab administered every six weeks can meaningfully enhance neoadjuvant chemotherapy efficacy in TNBC.
This study provides strong proof-of-concept that dose de-escalation of immunotherapy may preserve antitumor activity while dramatically reducing cost. In resource-constrained settings, this strategy has the potential to expand access to curative-intent immunotherapy, addressing one of the major inequities in global cancer care.
While longer follow-up is needed to confirm invasive disease-free and overall survival benefits, PLANeT introduces an important paradigm: immunotherapy dosing may not need to be “one size fits all” to achieve clinically meaningful benefit.
You can read all article here.


