Tripple negative breast cancer (TNBC) is an aggressive type of breast cancer that does not respond to hormonal or HER2-targeted treatments. For many years, chemotherapy was the only option. Recently, immune checkpoint inhibitors have changed how TNBC is treated, especially in early-stage and PD-L1-positive metastatic disease. Important clinical trials like KEYNOTE-522 and KEYNOTE-355 have led to new standards of care. This article reviews the major studies that brought immunotherapy into TNBC treatment and explains how these changes are helping patients today.
Title: Recent Advances in Immune Checkpoint Inhibitors for Triple-Negative Breast Cancer
Authors: Chiara Corti, Beyza Koca, Tasnim Rahman, Elizabeth A Mittendorf, Sara M Tolaney
Published in ImmunoTargets and Therapy
Background
Triple-negative breast cancer (TNBC) comprises 15–20% of all breast cancer cases and is defined by the lack of estrogen receptor (ER), progesterone receptor (PR), and HER2 expression. It is an aggressive subtype associated with a high recurrence rate and limited treatment options, making it a clinical challenge. Recent advances in immunotherapy, especially immune checkpoint inhibitors (ICIs) targeting the PD-1/PD-L1 pathway, have significantly altered the therapeutic landscape for TNBC. Due to the high presence of tumor-infiltrating lymphocytes (TILs), PD-L1 expression, and genomic instability in TNBC, it is considered more immunogenic than other breast cancer subtypes.
Methods
This review article analyzes data from major phase I–III clinical trials that have investigated the efficacy and safety of ICIs in both early-stage and metastatic TNBC. It discusses ICIs used as monotherapy or in combination with chemotherapy and highlights how these trials have contributed to shaping current treatment guidelines and FDA approvals.
These are the main trials mentioned in this study
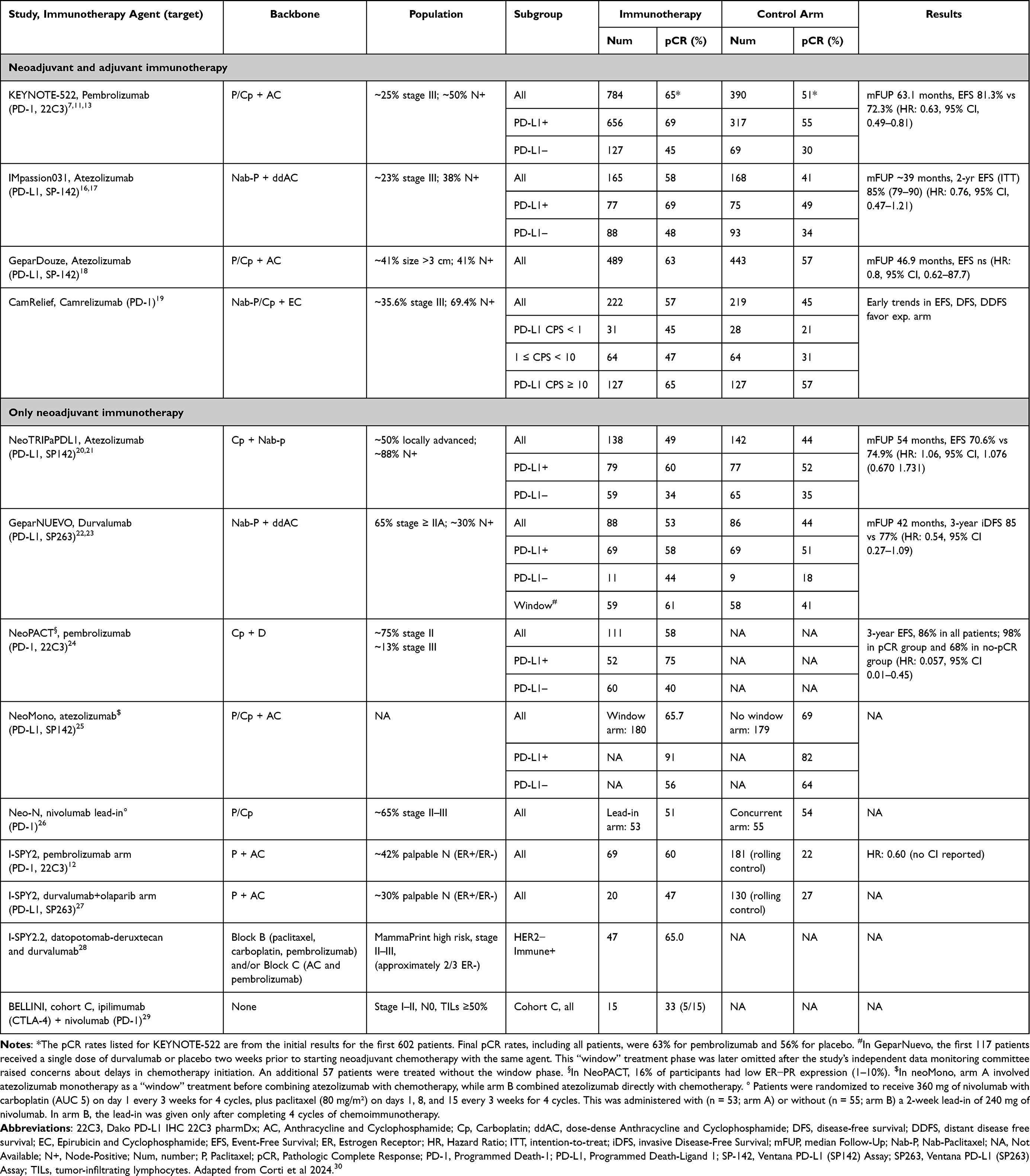
For Early-stage TNBC: Trials studying ICIs in the neoadjuvant setting (e.g., KEYNOTE-522, GeparNuevo, NeoTRIPaPDL1)
For Advanced/metastatic TNBC: Trials evaluating ICIs with chemotherapy (e.g., IMpassion130, KEYNOTE-355), and as monotherapy (e.g., KEYNOTE-119, IMpassion131)
Clinical Trials and Study Findings
KEYNOTE-522 (Phase III) – Early-Stage TNBC
KEYNOTE-522 established pembrolizumab plus chemotherapy as the standard of care for high-risk early-stage TNBC. The trial included over 1,100 patients with stage II–III TNBC. The neoadjuvant regimen combined pembrolizumab with paclitaxel and carboplatin, followed by anthracycline-based chemo, and continued pembrolizumab post-surgery.In March 2021 neoadjuvant + adjuvant pembrolizumab was approved by FDA
- pCR: 64.8% vs 51.2% (pembro vs placebo)
- Event-free survival (EFS) at 36 months: 84.5% vs 76.8%
GeparNuevo (Phase II)
This German study evaluated neoadjuvant durvalumab in early TNBC. Patients received a short “window-of-opportunity” phase of durvalumab monotherapy, followed by durvalumab + nab-paclitaxel and EC chemo. In this study the most benefits were observed in tumors with high TILs and PD-L1 expression
- pCR: Increased from 44.2% (placebo) to 53.4% (durvalumab)
NeoTRIPaPDL1 (Phase III)
This study tested atezolizumab + nab-paclitaxel/carboplatin in the neoadjuvant setting. Unlike KEYNOTE-522, anthracyclines were not part of the backbone. In this study no statistically significant improvement with atezolizumab was observed. Also this study raised questions about chemotherapy backbone impact on ICI synergy.
KEYNOTE-355 (Phase III) – Metastatic TNBC
This trial provided the basis for pembrolizumab + chemo as first-line therapy in PD-L1-positive (CPS ≥10) metastatic TNBC. In November 2020 FDA approved pembrolizumab in mTNBC with PD-L1 CPS ≥10
- PFS: 9.7 months vs 5.6 months (HR 0.65) in PD-L1+ group
- OS: 23.0 vs 16.1 months (HR 0.73) in PD-L1 CPS ≥10
IMpassion130 (Phase III)
This landmark study tested atezolizumab + nab-paclitaxel vs placebo + nab-paclitaxel in untreated metastatic TNBC.
PFS: 7.5 vs 5.0 months (HR 0.62) in PD-L1+ subgroup
- OS: 25.0 vs 15.5 months (HR 0.62) in PD-L1+ group (exploratory)
- Initially led to FDA accelerated approval of atezolizumab in PD-L1+ mTNBC
IMpassion131 (Phase III)
This follow-up study tested atezolizumab + paclitaxel instead of nab-paclitaxel. The trial failed to replicate the positive outcomes from IMpassion130. There was significant PFS difference between arms and FDA withdrawed atezolizumab approval in mTNBC in 2021.
KEYNOTE-119 (Phase III)
This trial evaluated pembrolizumab monotherapy vs chemotherapy (capecitabine, eribulin, etc.) in previously treated mTNBC. Also this studyshowed potential for monotherapy in highly PD-L1+ patients.
OS (overall): No significant difference (9.9 vs 10.8 months)
- In patients with PD-L1 CPS ≥20: OS improved to 14.9 vs 12.5 months (HR 0.58)
TONIC (Phase II)
A small Dutch study tested whether short-term induction therapy (e.g., radiation, chemo, or cyclophosphamide) could “prime” tumors for better response to ICIs.
Best response seen in patients pre-treated with doxorubicinSupports idea that tumor microenvironment modulation could improve ICI efficacy
Key Findings
The introduction of immune checkpoint inhibitors has significantly improved outcomes in selected TNBC patients. Pembrolizumab has emerged as the leading agent, validated by robust phase III trials. However, optimizing efficacy requires careful patient selection using validated biomarkers like PD-L1 CPS, attention to chemotherapy combinations, and potentially new strategies to modulate the tumor immune environment. Continued research is vital to extend benefits to a broader patient population and to integrate novel immunotherapeutic approaches.
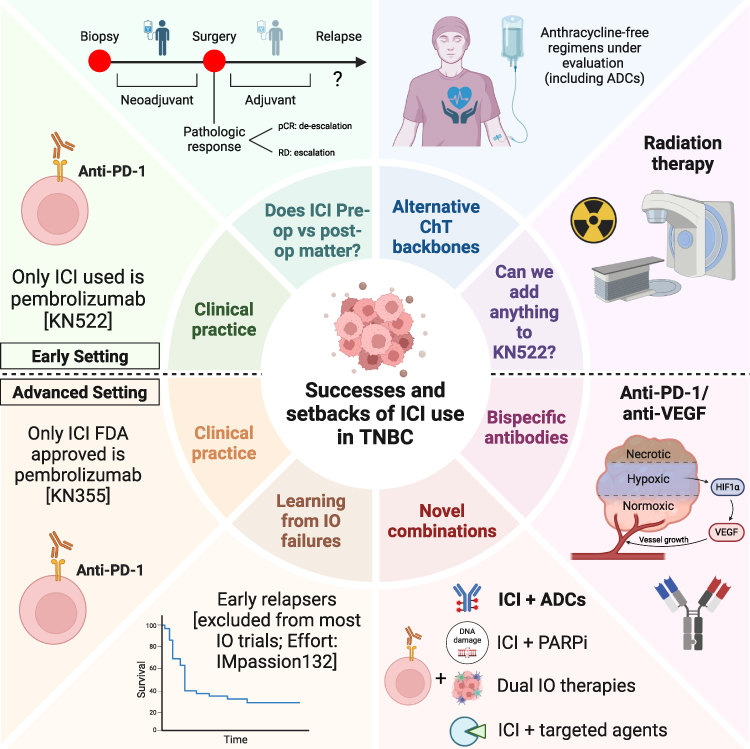
- Early-stage TNBC: Pembrolizumab + chemotherapy, based on KEYNOTE-522, is now standard for high-risk patients.
- Metastatic TNBC: Pembrolizumab + chemo is approved for PD-L1 CPS ≥10 (KEYNOTE-355).
- Atezolizumab is no longer approved due to inconsistent trial outcomes.
- Biomarkers matter: PD-L1 expression is critical in mTNBC. CPS ≥10 is the FDA threshold, but higher CPS (≥20) may indicate better outcomes.
- Chemotherapy backbone impacts efficacy: nab-paclitaxel seems more synergistic with ICIs than paclitaxel, possibly due to steroid premedication.
- Monotherapy with ICIs has limited benefit unless PD-L1 expression is high.
Key Takeaway Messages
- Pembrolizumab is the current standard of care for both early-stage and metastatic PD-L1+ TNBC.
- Atezolizumab, although initially promising, is not currently recommended for TNBC in the U.S. after the IMpassion131 results.
- Immunotherapy is more effective in earlier stages of disease when used as part of neoadjuvant combination therapy.
- Future trials should focus on improving patient selection, refining predictive biomarkers, and testing new ICI combinations (e.g., anti-CTLA-4, PARP inhibitors, or ADCs).
You can read the full article here.
Written by Sona Karamyan, MD


