Acquired resistance to PD-1/PD-L1 inhibitors is common in advanced NSCLC and is associated with poor outcomes. Strategies that reprogram an immunosuppressive tumor microenvironment while retaining PD-1 blockade and adding cytotoxic therapy may help recapture benefit. Sitravatinib (a multitarget TKI) was studied here as an immune-modulating partner for tislelizumab, with short-course docetaxel.
Methods and Study Design
- Design: Phase II, single-arm (no randomized control), multicenter
- Population: Advanced/metastatic NSCLC (stage IIIB/IV) with acquired resistance after prior PD-(L)1 therapy + platinum chemotherapy
- Treatment: tislelizumab + sitravatinib + docetaxel (docetaxel given for 2 cycles)
- Endpoints: primary = 6-month PFS rate; secondary = PFS, ORR, DCR, OS; safety
- Exploratory immune work: longitudinal TCR sequencing and single-cell secretome profiling to estimate T-cell polyfunctionality via PSI (polyfunctional strength index)
NSCLC
Results
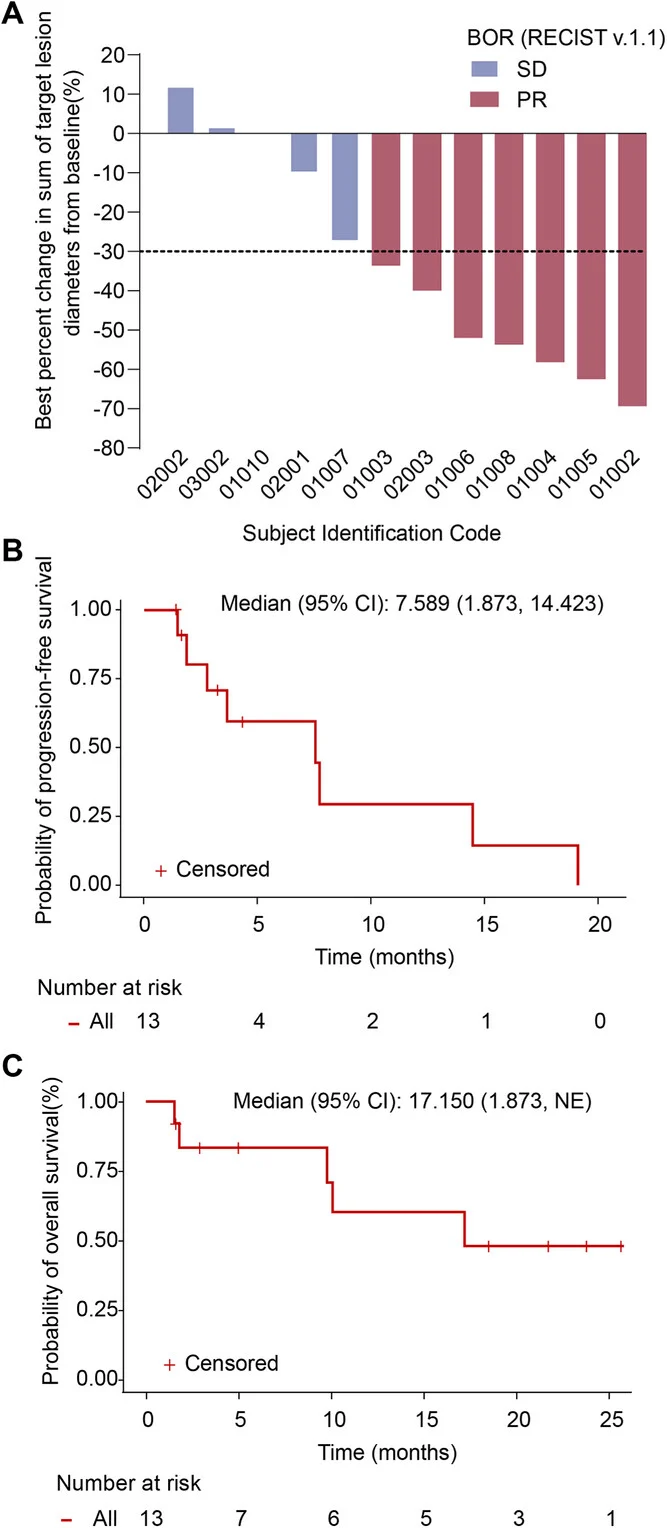
In this Phase II trial, 13 patients with advanced/metastatic NSCLC and acquired resistance to prior PD-(L)1 therapy were enrolled, and 12 were evaluable for tumor response. The median age was 56 years, most patients were men (84.6%), and histology was roughly evenly split between squamous and non-squamous disease. Importantly, every patient had previously received a PD-(L)1 inhibitor in combination with a platinum-based doublet chemotherapy regimen.
Clinical activity appeared encouraging in this heavily pretreated, post-ICI–resistance setting. Among the 12 evaluable patients, 7 achieved a partial response, translating to an objective response rate of 58.3%. The remaining patients achieved stable disease, resulting in a disease control rate of 100%. Median progression-free survival was 7.6 months, and median overall survival reached 17.2 months. While these outcomes are numerically strong for a population with acquired resistance to immunotherapy, they should be interpreted cautiously given the very small sample size and the single-arm design without a randomized comparator.
Treatment-related toxicity was common and often severe. All patients experienced at least one treatment-related adverse event, and grade ≥3 events occurred in 92.3% of participants. The most frequent severe toxicities were hematologic—particularly neutropenia and leukopenia—consistent with the expected risk profile of docetaxel-containing regimens. Immune-related adverse events were also observed, affecting roughly one-third of patients.
Exploratory immune signals (hypothesis-generating)
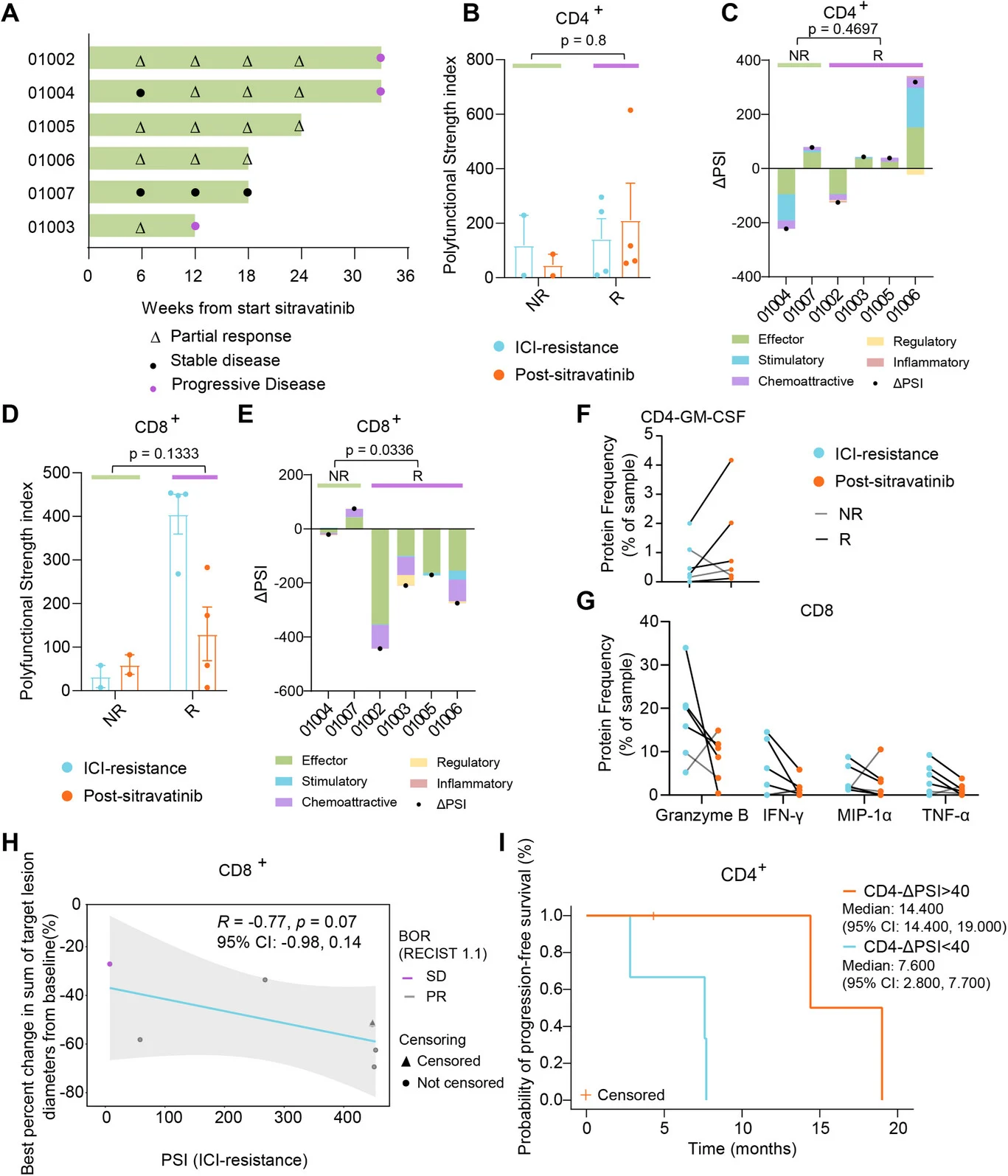
Exploratory immune analyses, which should be regarded as hypothesis-generating, suggested dynamic changes in T-cell biology following treatment. Compared with treatment-naïve reference samples, patients with ICI-resistant disease appeared to have reduced T-cell receptor (TCR) diversity, consistent with an immunologically constrained state. After initiation of the sitravatinib-based combination, a trend toward increased TCR diversity was observed, although this finding was not definitive given the limited sample size.
Functional analyses further revealed divergent patterns between CD8⁺ and CD4⁺ T-cell compartments. Higher baseline polyfunctional strength index (PSI) in CD8⁺ T cells tended to correlate with greater tumor shrinkage and earlier clinical response, suggesting that preserved CD8⁺ functional capacity at baseline may predispose patients to benefit from therapy. In contrast, on-treatment changes in CD4⁺ T-cell function appeared more closely linked to disease control over time: patients who exhibited larger increases in CD4⁺ PSI (ΔPSI) experienced longer progression-free survival in exploratory analyses.
Collectively, these observations imply that the therapeutic regimen may exert immunomodulatory effects beyond the cytotoxic contribution of chemotherapy alone, potentially reshaping T-cell repertoire diversity and function. However, given the exploratory nature of these analyses and the small cohort size, these findings require confirmation in larger, prospective studies.
NSCLC
Key Findings
- The triplet produced high response rates and encouraging PFS/OS in a difficult population (post-PD-(L)1 acquired resistance).
- Toxicity was substantial, mainly hematologic—important for real-world feasibility.
- Immune correlative work suggests TCR/functional T-cell remodeling, with potentially different roles for CD8 baseline function vs CD4 on-treatment changes.
Key Takeaway Messages
- Promising activity in PD-(L)1–resistant NSCLC: ORR ~58%, median PFS ~7.6 months (small cohort).
- Toxicity is a major constraint (very high grade ≥3 AEs), likely driven by docetaxel plus combination intensity.
- Biomarker hints: baseline CD8 function and on-treatment CD4 functional shifts may relate to benefit—but this is not practice-changing yet.
- Because this is single-arm, n=13, and the program ended early (drug development discontinuation), it should be viewed as signal-finding, not confirmatory.
Conclusion
This Phase II study suggests that combining a microenvironment-modulating TKI with PD-1 blockade and short-course chemotherapy can generate meaningful responses after acquired PD-(L)1 resistance in advanced NSCLC. However, the evidence remains hypothesis-generating due to very small sample size, lack of a control arm, and tolerability concerns. Prospective, controlled validation (with more scalable agents/safer dosing) is essential.
Read all article here