For high-risk, resected locally advanced head and neck squamous cell carcinoma (LA-SCCHN), postoperative radiotherapy with concomitant high-dose cisplatin has been the standard approach for ~20 years, yet 40–45% of patients still relapse within ~2 years. The NIVOPOST-OP trial tested whether adding PD-1 blockade (nivolumab) to postoperative cisplatin-based chemoradiotherapy could improve outcomes in this high-risk population.
NIVOPOST-OP
Methods and Study Design
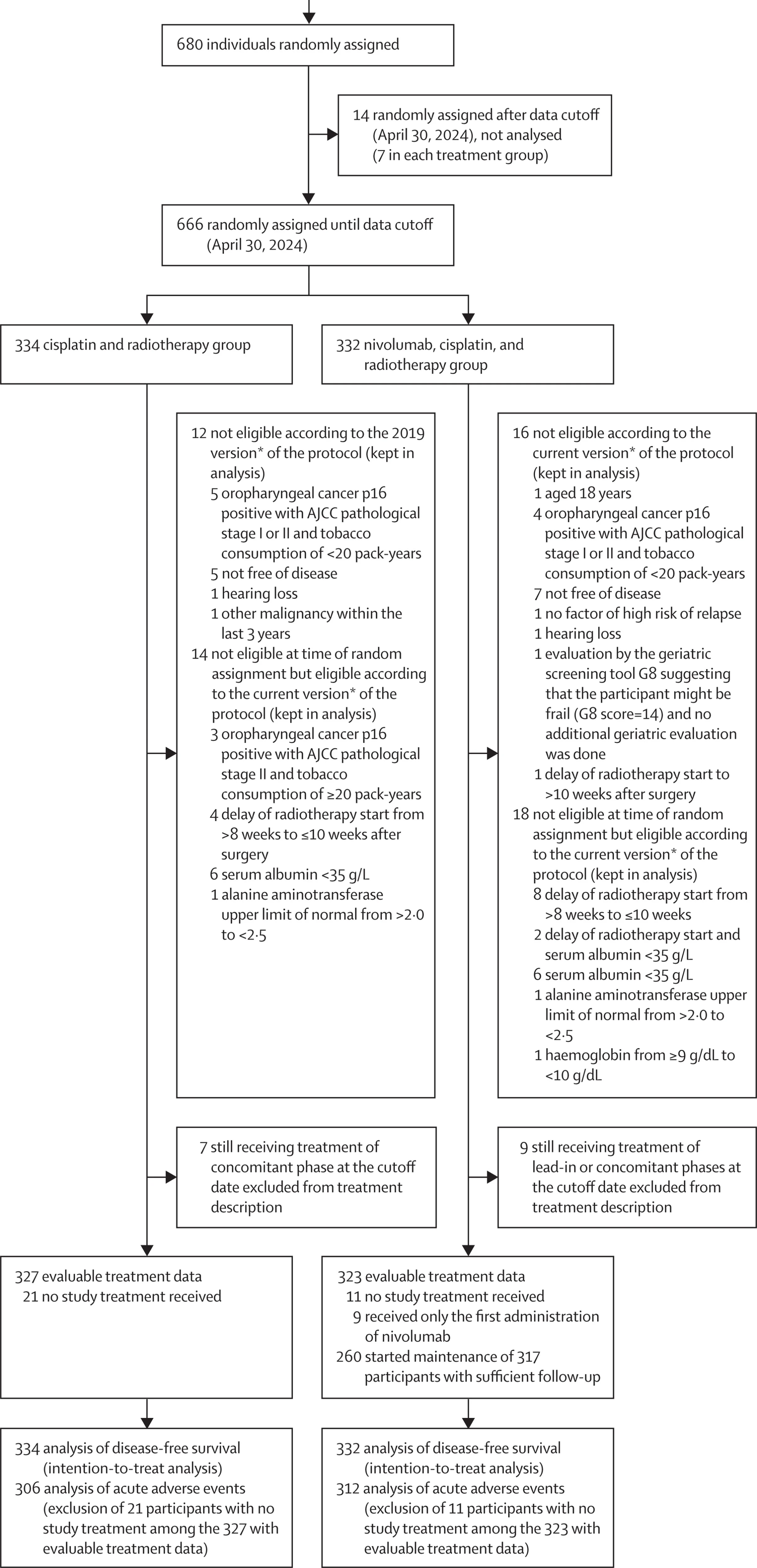
NIVOPOST-OP (GORTEC 2018-01) was an international, multicenter, randomized, open-label phase 3 trial across 82 sites in six European countries. Eligible patients (age 19–74, ECOG 0–1) had SCC of the oral cavity, oropharynx, larynx, or hypopharynx, underwent macroscopically complete resection (R0/R1), and were disease-free at randomization with ≥1 high-risk pathological feature:
- Nodal extracapsular extension (ECE)
- Microscopically positive margin (R1) or close margin ≤1 mm
- ≥4 cervical nodes involved without ECE
- Multiple perineural invasions
Patients were randomized 1:1 to:
- Control: postoperative IMRT 66 Gy/33 fractions + cisplatin 100 mg/m² q3w ×3
- Experimental: nivolumab 240 mg lead-in (≥2 weeks post-op), then the same cisplatin-RT with concurrent nivolumab 360 mg q3w ×3, followed by adjuvant nivolumab 480 mg q4w ×6 (overall ~6 months planned)
Primary endpoint: investigator-assessed disease-free survival (DFS) in the intention-to-treat population.
Planned DFS effect size targeted HR 0.65 with 230 DFS events required for primary analysis. Median follow-up at cutoff was 30.3 months.
Results
Across 82 sites in six European countries, 680 patients with high-risk, resected LA-SCCHN were randomized between Oct 15, 2018 and July 3, 2024. The primary DFS analysis was triggered at the prespecified cutoff (April 30, 2024) and included 666 patients randomized before that date, with 252 disease-free survival events recorded.
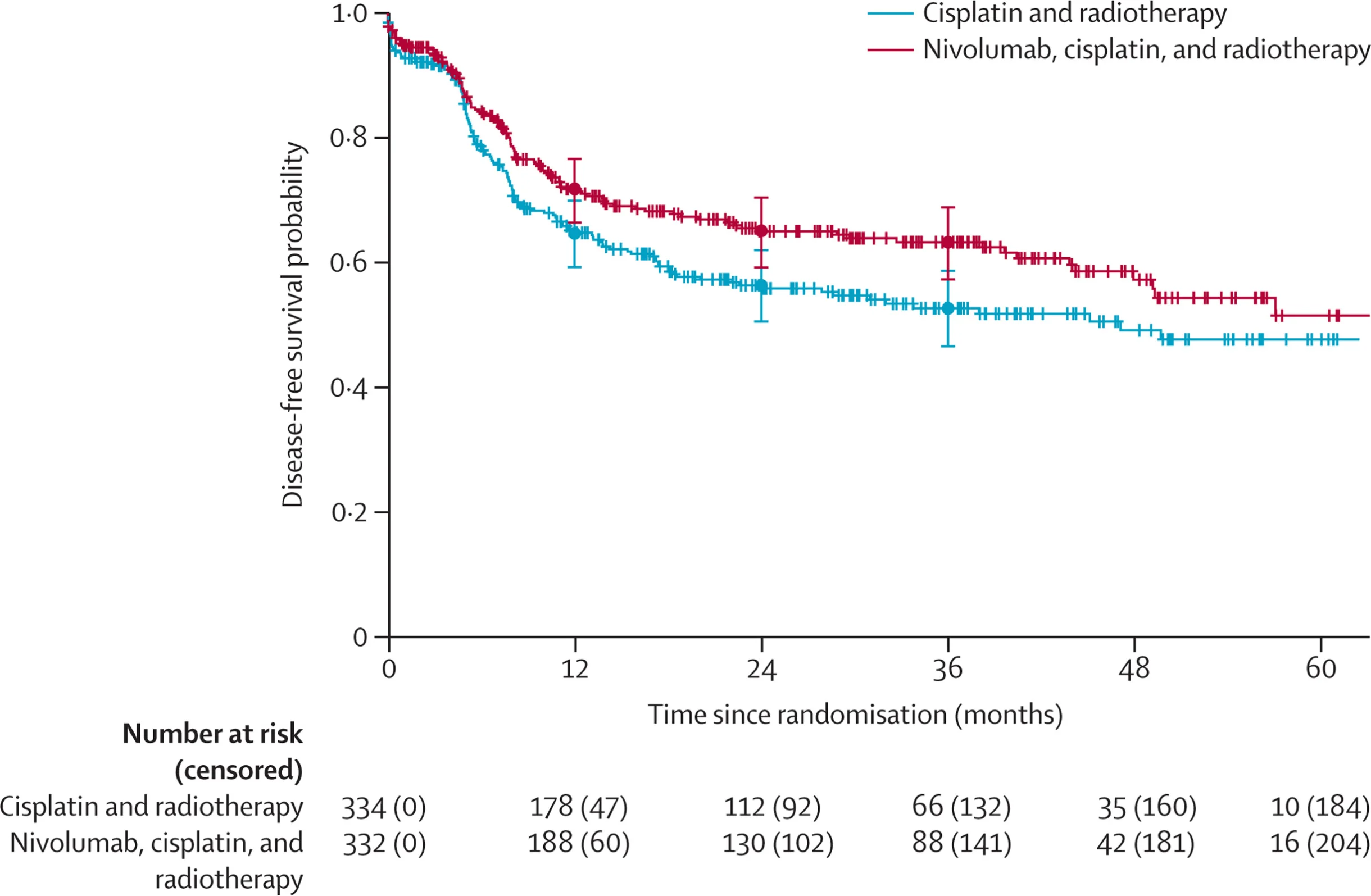
Adding nivolumab to standard postoperative cisplatin-based chemoradiotherapy produced a statistically significant DFS benefit. The risk of relapse or death was reduced by about one quarter (HR 0.76, 95% CI 0.60–0.98; p=0.034), translating into a clinically meaningful separation of the curves: 3-year DFS 63.1% with nivolumab versus 52.5% with cisplatin-RT alone.
When the components of DFS were unpacked, the improvement was predominantly locoregional. The cumulative incidence of locoregional relapse was lower in the nivolumab arm (subdistribution HR 0.63, 95% CI 0.42–0.94), whereas distant relapse occurred at similar rates between groups (subdistribution HR approximately 0.95), suggesting the main added value of nivolumab in this setting is better local–regional control rather than a clear impact on metastatic spread at this follow-up.
Importantly, PD-L1 CPS did not function as a predictive biomarker here: the DFS benefit was consistent across PD-L1 strata in prespecified subgroup analyses, with no meaningful interaction signal.
At the time of the report, overall survival data were still immature, so the study was not yet positioned to make a definitive OS claim beyond noting that longer follow-up is needed for the planned analysis.
NIVOPOST-OP
Safety
Adding nivolumab increased toxicity modestly:
- Grade 4 treatment-related AEs: 10% vs 5% (nivolumab vs control)
- Treatment-related deaths: 2 in each group
- More treatment-related serious AEs in the nivolumab arm (notably some increases in renal events and thyroid disorders, consistent with PD-1 effects plus cisplatin risk)
Longer-term (post-9 months) severe treatment-related AEs were uncommon, with no grade 4 treatment-related events reported in that later period.
Key Findings
- Nivolumab + postoperative cisplatin-RT significantly improved DFS in high-risk resected LA-SCCHN.
- The gain was primarily locoregional control, not distant metastasis reduction.
- Benefit did not depend on PD-L1 CPS, supporting broad applicability in this high-risk postoperative population.
- Toxicity increased but remained acceptable, without excess treatment-related mortality.
Conclusion
In resected, high-risk LA-SCCHN, adding nivolumab to standard postoperative cisplatin-based chemoradiotherapy improved DFS with a moderate toxicity increase and no rise in treatment-related deaths, supporting nivolumab + cisplatin-RT as a new postoperative standard option for this population.
You Can Read All Article Here