The ESMO 2025 Congress is set to feature groundbreaking studies in the field of immunotherapy, with a focus on head and neck cancers (HNSCC) and endocrine tumors such as neuroendocrine carcinomas (NEC) cancers. This article provides an in-depth look at the top clinical trials in immuno-oncology that will be presented at ESMO 2025, offering a glimpse of what to expect and why each of these trials could significantly impact future treatment strategies.
Immunotherapy has rapidly transformed cancer treatment, particularly in head and neck cancers, where checkpoint inhibitors like PD-1 and PD-L1 antibodies have shown promising results. This year’s ESMO congress will showcase studies that go beyond PD-1, exploring innovative combinations with chemotherapy, radiotherapy, and novel immune targets. For thyroid cancers and other endocrine malignancies, trials evaluating targeted immunotherapies aim to refine treatment paradigms and improve patient outcomes. Below, we dive into the key trials to watch for their mechanisms of action, patient populations, and what is currently known about each study’s results.
ZG006: Trispecific T-cell Engager in Refractory Neuroendocrine Carcinoma (NEC)
Abstract: 1707MO
Presenter: Chuanhua Zhao
Session: Mini Oral – NETs & Endocrine Tumours, Mon 20.10.2025, 08:30–10:00 (Koblenz Auditorium)
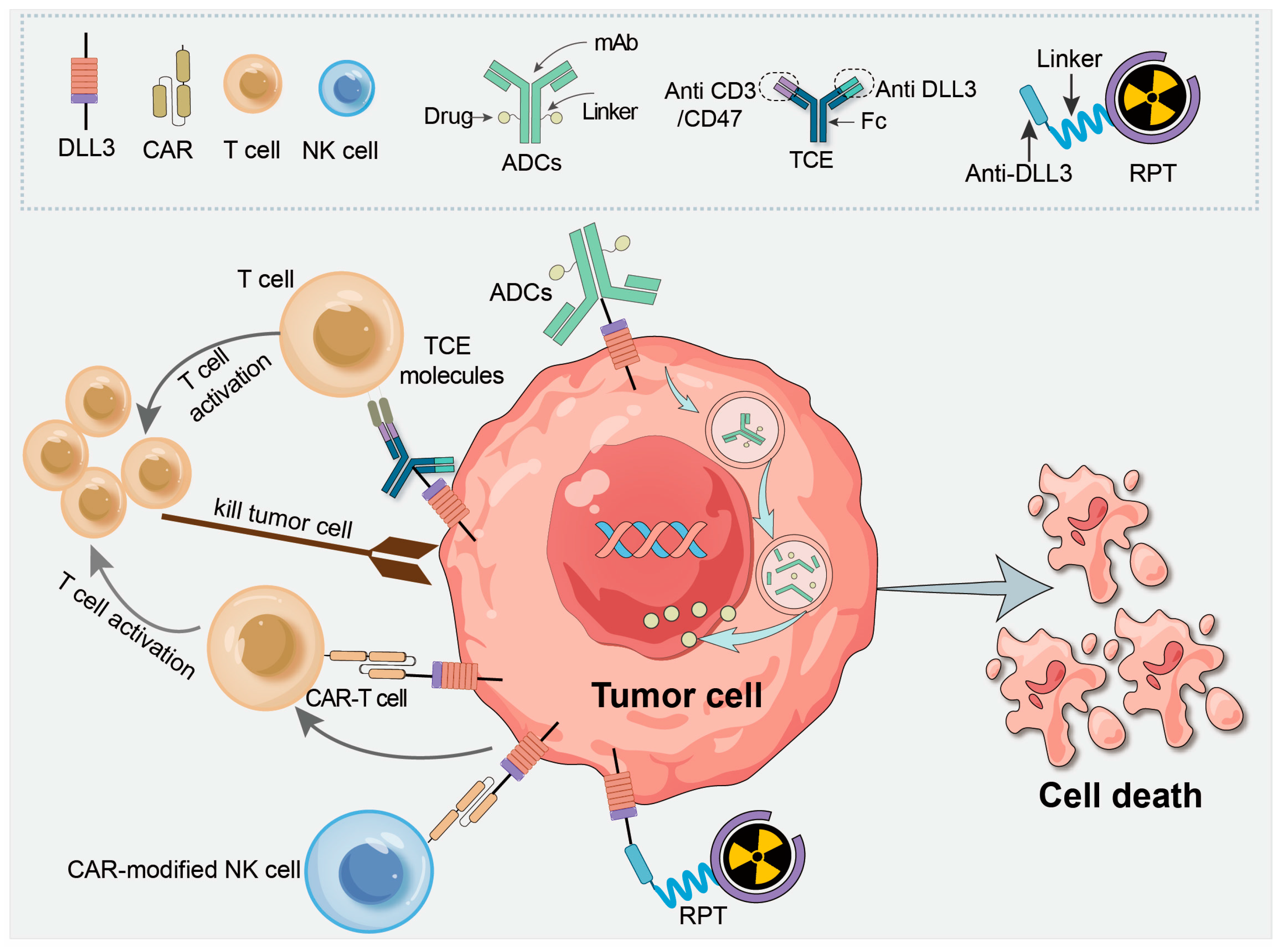
ZG006 is a trispecific T-cell engager that targets DLL3, a tumor-associated antigen found in high expression in neuroendocrine carcinomas. By engaging both DLL3 and CD3, ZG006 enhances T-cell cytotoxicity, specifically targeting DLL3-expressing tumor cells. It is being tested in patients with platinum-refractory neuroendocrine carcinoma (NEC), a group typically resistant to standard chemotherapy and immune checkpoint inhibitors.
Source: Wang et al, Pharmaceutics 2025, 17(4), 520; https://doi.org/10.3390/pharmaceutics17040520
What’s Known?
This phase 2 study explores the safety, pharmacodynamics, and efficacy of ZG006 in patients with refractory NEC. At ASCO 2025, preliminary results from the Phase 2 trial of ZG006 in advanced neuroendocrine carcinoma showed a 33.3% objective response rate (ORR) and 66.7% disease control rate (DCR), with manageable treatment-related adverse events (TRAEs) such as pyrexia and cytokine release syndrome. The trial is assessing objective response rates (ORR) and progression-free survival (PFS) as key endpoints.
CompARE: Neoadjuvant Durvalumab Plus Chemoradiotherapy vs Chemoradiotherapy Alone in High-Risk Oropharyngeal Cancer (OPC)
Abstract: 1317O
Presenter: Hisham M. Mehanna
Session: 10:15–10:25
This phase III trial investigates the combination of durvalumab, a PD-L1 inhibitor, with chemoradiotherapy (CRT) in patients with intermediate- to high-risk oropharyngeal cancer (OPC). Durvalumab works by blocking the interaction between PD-L1 on tumor cells and PD-1 on immune cells, thereby reactivating T-cell responses against the tumor. The study aims to assess the benefit of neoadjuvant immunotherapy in combination with CRT to improve patient outcomes.
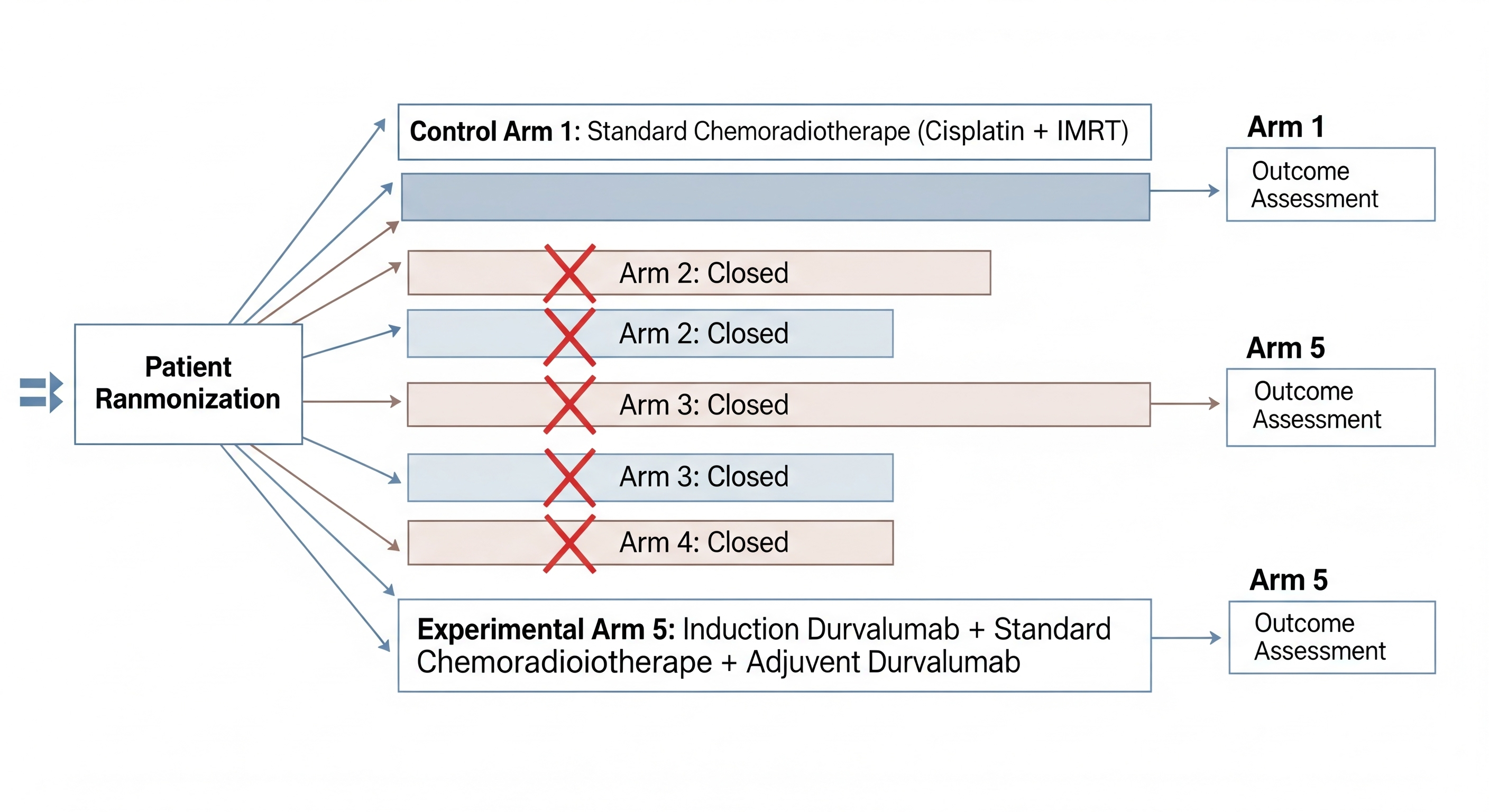
Study Scheme Created by OncoDaily and Google Gemini
The trial is focused on high-risk OPC patients, particularly those with HPV-negative tumors, who traditionally have poorer outcomes with standard CRT alone. Early results suggest improved event-free survival (EFS) with the addition of durvalumab, though final results are awaited for overall survival (OS) benefits.
CAMORAL: Perioperative Camrelizumab Plus Chemotherapy in Locally Advanced Head and Neck Squamous Cell Carcinoma (HNSCC)
Abstract: 1319O
Presenter: Yue He
Session: 10:55–11:05
Camrelizumab is a PD-1 inhibitor tested in combination with chemotherapy as a perioperative treatment for locally advanced squamous cell carcinoma of the head and neck (HNSCC). This phase II study evaluates the potential of combining immunotherapy with chemotherapy to improve pathological response and disease-free survival (DFS) in patients undergoing surgery.
The trial explores how adding camrelizumab before and after surgery (perioperative setting) might enhance tumor immunogenicity and reduce recurrence. Preliminary data suggests encouraging rates of major pathological response (MPR), with low-grade toxicities observed. The final analysis will clarify its role in clinical practice and long-term outcomes.
ADRISK: Adjuvant Cisplatin-Based Radiochemotherapy ± Pembrolizumab in Locally Advanced HNSCC
Abstract: 1320O
Presenter: Andreas Dietz
Session: 11:05–11:15
This trial evaluates the combination of cisplatin-based radiochemotherapy (aRCH) with or without pembrolizumab, an anti-PD-1 monoclonal antibody, in locally advanced head and neck squamous cell carcinoma (HNSCC). Pembrolizumab works by inhibiting PD-1, enhancing immune recognition and destruction of tumor cells. The study aims to determine if adding pembrolizumab improves disease-free survival (DFS) and recurrence rates compared to radiochemotherapy alone.
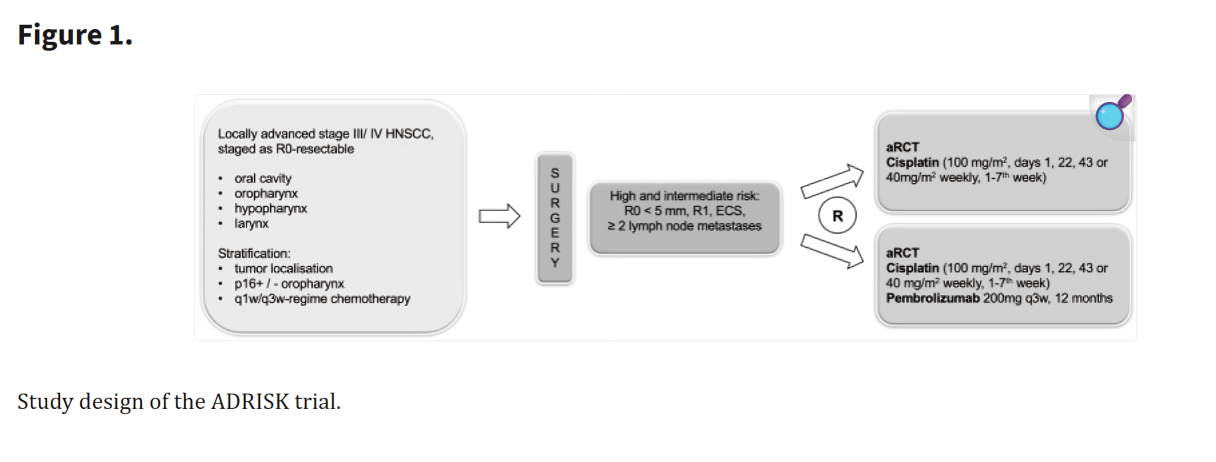
Source: Wiegand et al, Frontiers in Oncology
This study marks a significant step in testing adjuvant immunotherapy in HNSCC, a setting where recurrence rates remain high. Early data suggest that immune checkpoint inhibition post-surgery may reduce micrometastases and delay recurrence, though long-term survival data are still pending.
Retifanlimab (Anti-PD-1) ± Anti-LAG-3 ± Anti-TIM-3 in Recurrent/Metastatic PD-L1+ HNSCC
Abstract: 1325MO
Presenter: Robert Haddad
Session: 16:40–16:45
Drug Mechanism & Population:
This trial examines the combination of retifanlimab (an anti-PD-1 antibody) with anti-LAG-3 and anti-TIM-3 antibodies in PD-L1+ recurrent/metastatic HNSCC. The dual blockade of LAG-3 and TIM-3, co-inhibitory receptors that suppress immune responses, aims to further enhance the immune system’s ability to target and kill cancer cells.
In this double-blind, randomized phase 2 trial, early findings indicate that combination therapy may significantly enhance immune responses compared to PD-1 monotherapy, offering a potential new strategy for tumors that are resistant to current checkpoint inhibitors. The trial will evaluate ORR and PFS, along with immune-related adverse events (irAEs). At ASCO 2025 study invetsigators demonstrated numerically longer median PFS (~5–6 mo) versus PD-1 monotherapy (~3 mo), supporting enhanced activity of concurrent checkpoint inhibition in PD-L1+ SCCHN.
SKYSCRAPER-09: Tiragolumab + Atezolizumab vs Atezolizumab in PD-L1+ R/M SCCHN
Abstract: 1348MO
Presenter: Amanda Psyrri
Session: 16:45–16:50
Drug Mechanism & Population:
Tiragolumab, a TIGIT inhibitor, is being tested in combination with atezolizumab (an anti-PD-L1 monoclonal antibody) in recurrent/metastatic squamous cell carcinoma of the head and neck (R/M SCCHN). TIGIT is an immune checkpoint that downregulates T-cell function; targeting it in combination with PD-L1 inhibition could enhance anti-tumor immunity.
Preliminary data suggests that TIGIT blockade may significantly enhance response rates compared to PD-L1 alone. The combination has shown promising safety profiles, with manageable adverse events. The study will explore efficacy, with a focus on improving PFS in PD-L1+ patients.
KEYNOTE-689: Neoadjuvant-Adjuvant Pembrolizumab + SOC in Resectable Locally Advanced HNSCC
Abstract: 1330MO
Presenter: Yungan Tao
Session: 17:10–17:15
Drug Mechanism & Population:
This study examines pembrolizumab, an anti-PD-1 agent, in combination with standard-of-care therapy in resectable locally advanced HNSCC. The study aims to evaluate patient-reported outcomes (PRO) to determine the impact of immunotherapy on quality of life and functional recovery.
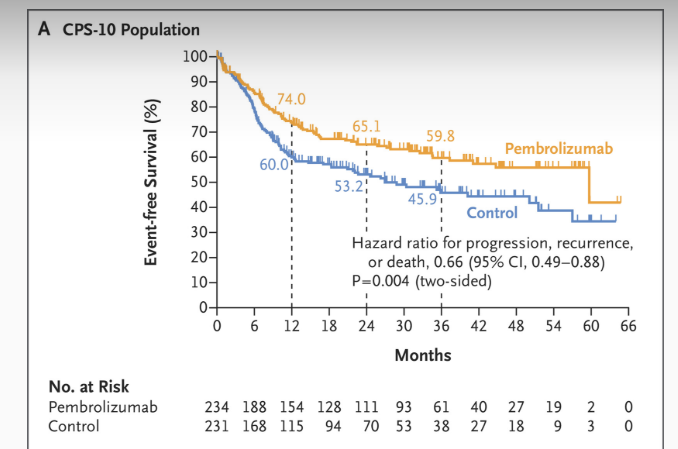
Figure: Event-free Survival as Assessed by Central Review. Source:Uppaluri et a, 2025, NEJM
The KEYNOTE-689 trial is critical for understanding how neoadjuvant immunotherapy can affect functional recovery post-surgery. In this phase 3 trial, adding perioperative pembrolizumab (2 cycles neoadjuvant + 15 cycles adjuvant) to standard care significantly improved outcomes: 3-year event-free survival (EFS) was 59.8% vs 45.9% in PD-L1 CPS ≥10 (HR 0.66, 95% CI 0.49–0.88; p=0.004) and 57.6% vs 46.4% in the total population (HR 0.73, 95% CI 0.58–0.92; p=0.008). Major pathologic response occurred in ~9% with pembrolizumab vs 0% with SOC. Safety was consistent with prior PD-1 experience, with grade ≥3 TRAEs in 44.6% vs 42.9%. Patient-reported outcomes suggested improved quality-of-life recovery post-surgery, underscoring the potential of perioperative PD-1 blockade to meaningfully alter the natural history of resectable locally advanced HNSCC.
Amivantamab in Recurrent/Metastatic HNSCC After IO and Chemotherapy Progression
Abstract: 1327MO
Presenter: Kevin J. Harrington
Session: 17:28–17:33
Drug Mechanism & Population:
Amivantamab is an EGFR/MET bispecific antibody that targets EGFR and MET to overcome resistance in HNSCC that has progressed after checkpoint inhibition and chemotherapy.
Preliminary findings suggest that amivantamab has the potential to induce objective responses in patients with EGFR/MET-driven resistance, showing a favorable safety profile. The trial will assess response rates and survival in a heavily pretreated population.
KEYNOTE-717: Hypofractionated RT + Pembrolizumab in R/M HNSCC
Abstract: 1328MO
Presenter: Markus Hecht
Session: 17:10–17:15
This trial explores the combination of hypofractionated radiotherapy with pembrolizumab in patients with recurrent/metastatic HNSCC. The goal is to test if radiotherapy-induced immune activation can improve the efficacy of PD-1 therapy in advanced cases.
The study’s primary endpoint is to assess systemic response rates (ORR), with additional endpoints evaluating the abscopal effect—where local tumor irradiation induces immune responses in distant metastases. Initial results show promising immune activation signatures.
EV-202: Enfortumab Vedotin + Pembrolizumab in 1L R/M HNSCC
Abstract: 1329MO
Presenter: Paul L. Swiecicki
Session: 17:38–17:43
Enfortumab vedotin (EV) is an ADC targeting Nectin-4, combined with pembrolizumab, to treat recurrent/metastatic HNSCC. EV works by delivering a potent cytotoxic payload directly to tumor cells expressing Nectin-4.
Read more about Enfortumab Vedotin on OncoDaily
The combination is being tested as a first-line treatment in PD-L1+ HNSCC. Preliminary findings suggest good response rates and manageable toxicity, especially in tumors expressing Nectin-4. Further data will be crucial to establish its place in frontline therapy. At ASCO 2025 (TPS6116): the ongoing EV-202 R/M HNSCC cohort is testing enfortumab vedotin + pembrolizumab 1L in CPS ≥ 1 with a go at ≥5/20 interim responders and promising at ≥14/40 total responders (primary ORR), contextualized by EV monotherapy ORR 23.9% and KEYNOTE-048 1L pembrolizumab median OS 12.3 mo.
The ESMO 2025 Congress is set to provide vital insights into the next frontier of immunotherapy for head and neck cancers and endocrine tumors. From checkpoint inhibitors to TCEs and ADCs, these trials offer promising strategies to expand treatment options for patients with difficult-to-treat cancers. For each study, careful attention to trial design, patient population, and early data will help clinicians assess the future role of these therapies in clinical practice.

Read Also TOP IO Trials in Lung Cancer to Watch at ESMO 2025