The NATALEE Trial 5-Year Analysis at ESMO 2025 (CLEE011O12301C) continues to redefine the adjuvant treatment landscape for hormone receptor–positive (HR+), HER2-negative early breast cancer (EBC). Presented with mature 5-year efficacy data, the study demonstrates that adjuvant ribociclib (RIB) combined with a nonsteroidal aromatase inhibitor (NSAI) significantly improves invasive disease-free survival (iDFS) compared to NSAI alone, with sustained benefit across subgroups and a favorable long-term safety profile.
The NATALEE Trial 5-Year Analysis at ESMO 2025 was designed to evaluate whether extending CDK4/6 inhibition into the early-stage setting could meaningfully reduce recurrence risk in a broader patient population than previous adjuvant trials. The new data, reported at ESMO 2025, confirm that the combination of RIB + NSAI not only delays recurrence but continues to show a positive trend toward overall survival (OS), reinforcing the long-term benefit and durable efficacy of adjuvant ribociclib in early breast cancer.
Methods
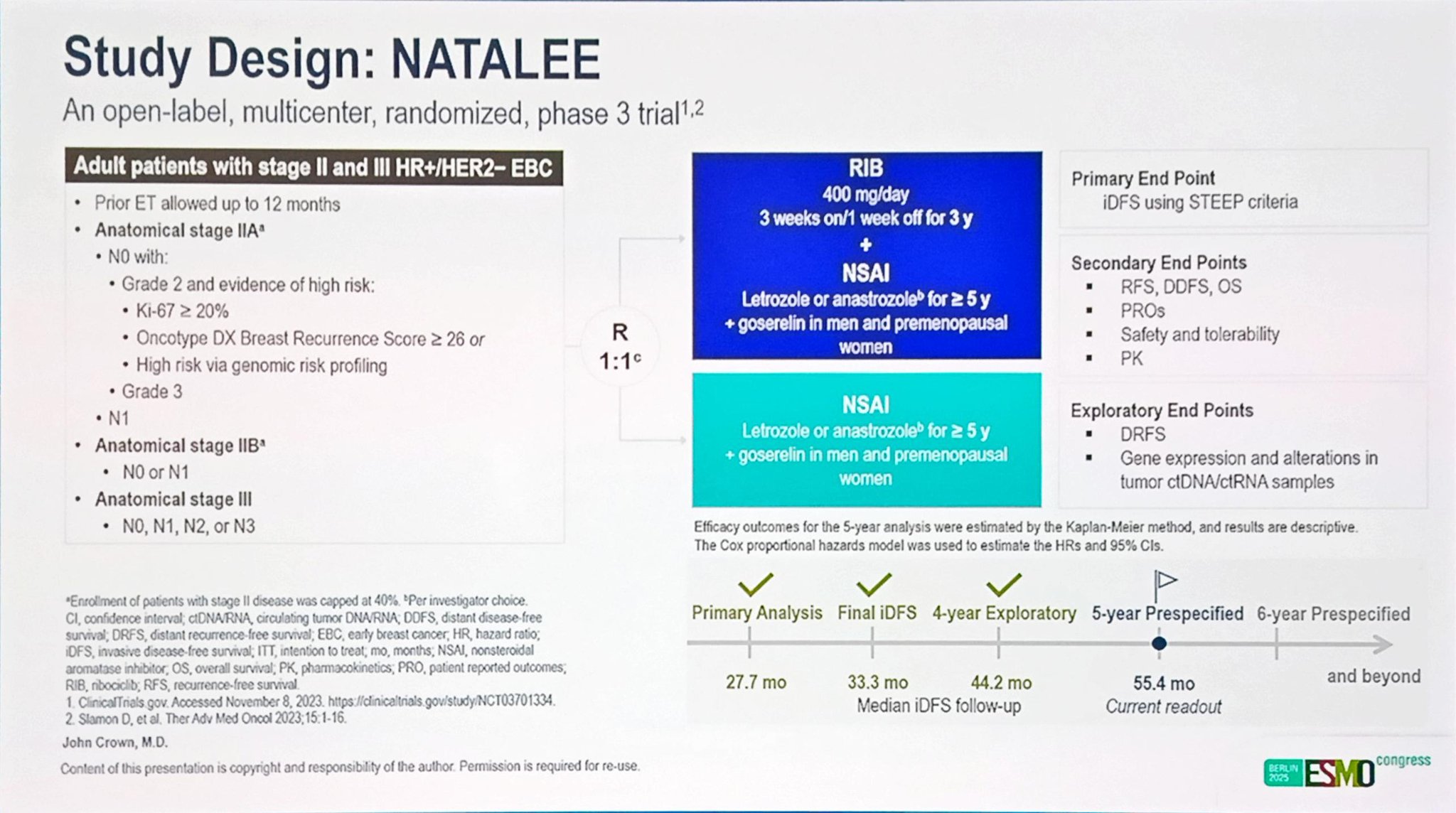
The NATALEE trial enrolled patients with stage II and III HR+/HER2− early breast cancer, including those with node-negative (N0) disease at high clinical risk of recurrence. Participants were randomized 1:1 to receive either RIB (400 mg daily, 3 weeks on/1 week off for 3 years) in combination with NSAI therapy—letrozole (2.5 mg daily) or anastrozole (1 mg daily) for five years—or NSAI alone.
Male patients and premenopausal women received goserelin for ovarian function suppression. Eligible patients included those with anatomical stage IIA (N1 [1–3 axillary lymph nodes] or N0 with additional high-risk factors), as well as stage IIB and stage III disease, per AJCC 8th edition criteria.
The primary endpoint was invasive disease-free survival (iDFS). Secondary endpoints included distant disease-free survival (DDFS), distant relapse-free survival (DRFS), and overall survival (OS). Time-to-event analyses were conducted using Kaplan–Meier methods, and between-group comparisons were made via the stratified log-rank test.
Results
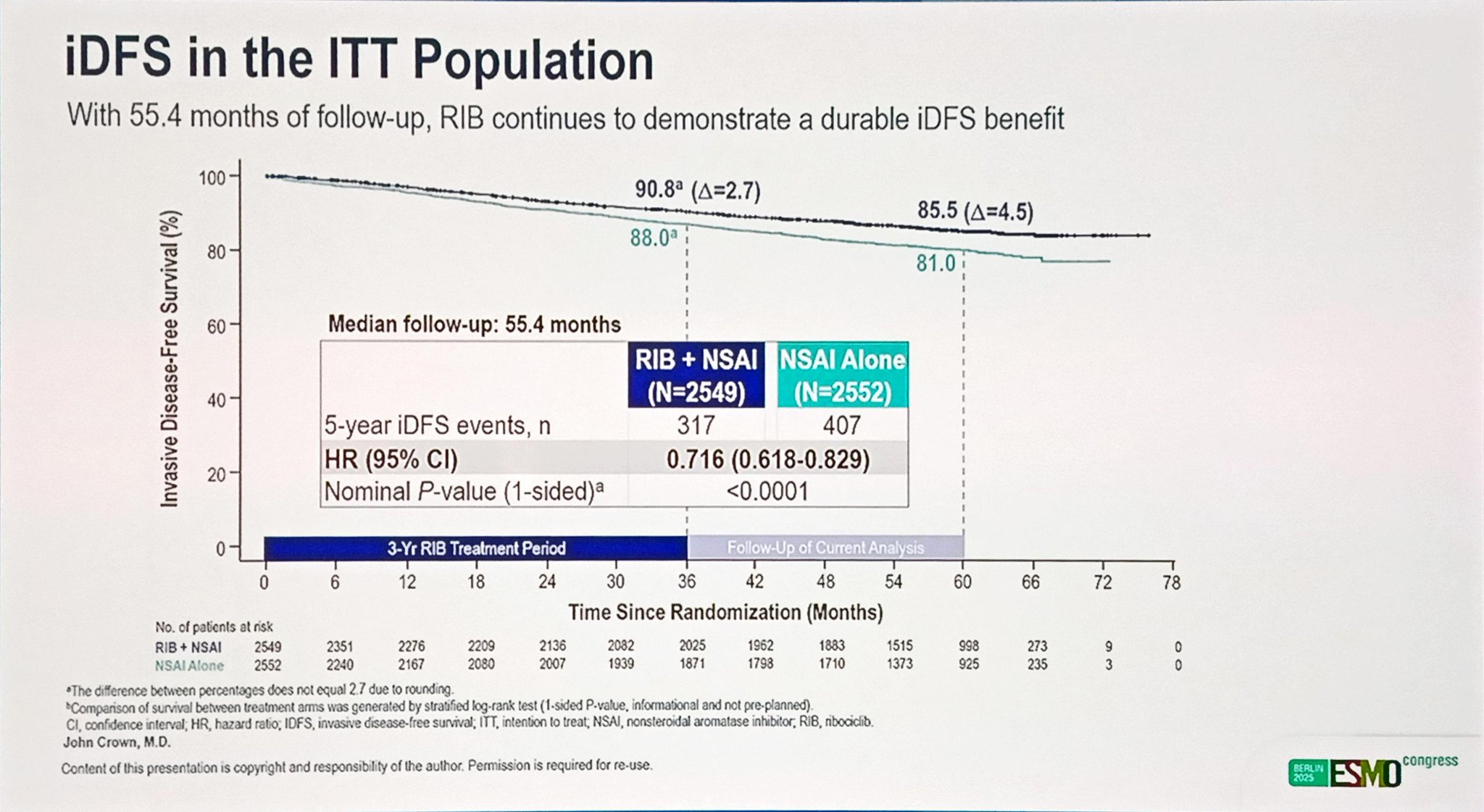
At the data cutoff (May 28, 2025), all patients had completed the ribociclib treatment phase, and approximately 36.5% of patients in the RIB + NSAI arm and 34.4% in the NSAI-alone arm had completed the planned five years of endocrine therapy. The median follow-up for iDFS was 55.4 months.
The addition of ribociclib to NSAI produced a persistent and clinically meaningful iDFS benefit, with a hazard ratio (HR) of 0.716 (95% CI: 0.618–0.829; nominal 1-sided P < .0001). This translates to a 28.4% relative reduction in the risk of invasive disease recurrence compared to NSAI alone.
The absolute iDFS rates favored ribociclib across all time points:
- 3 years: 90.8% (RIB + NSAI) vs 88.0% (NSAI alone)
- 4 years: 88.3% vs 83.9%
- 5 years: 85.5% vs 81.0%
These figures represent absolute iDFS improvements of 2.7%, 4.4%, and 4.5%, respectively, showing that the magnitude of benefit increased with longer follow-up.
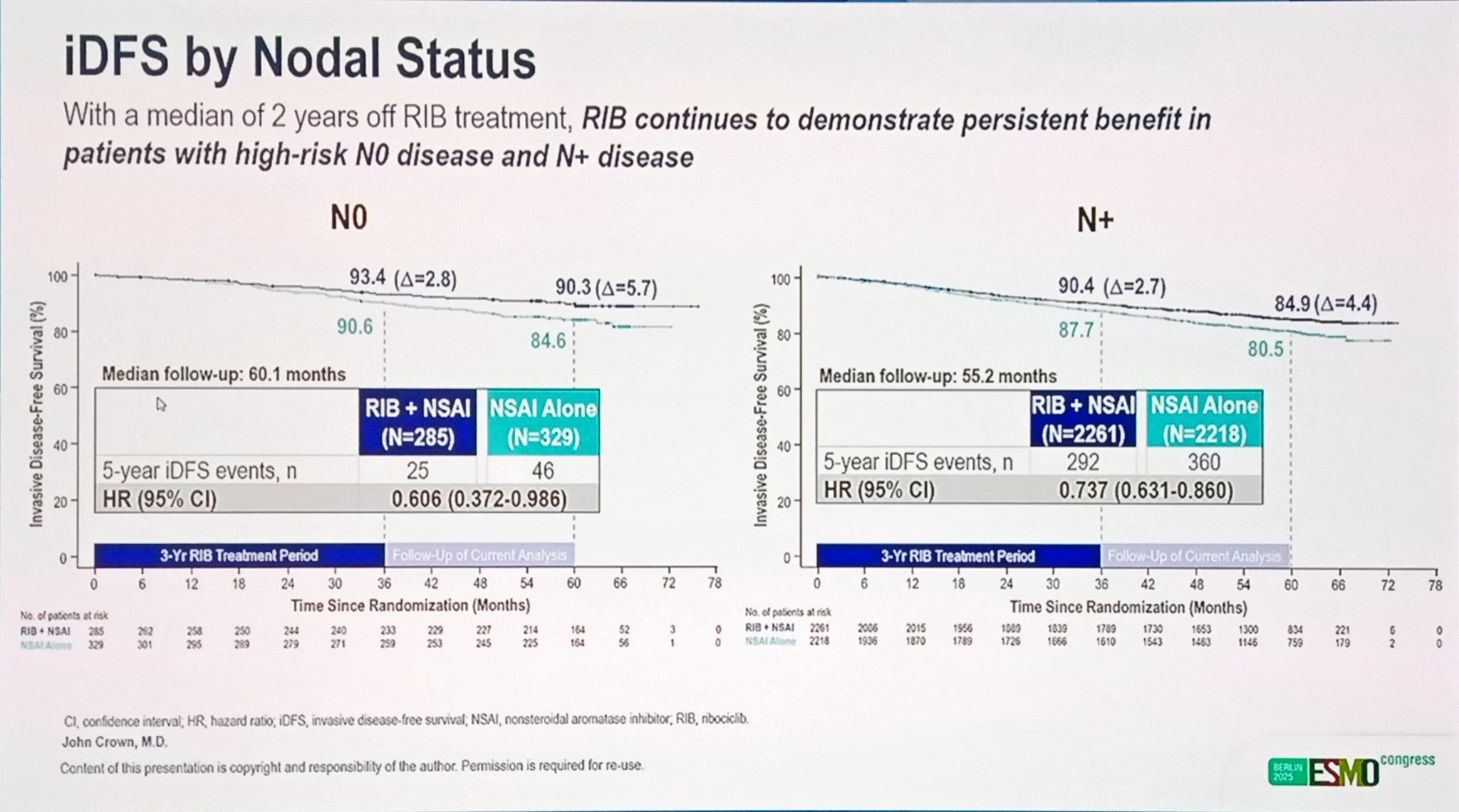
Importantly, the iDFS advantage extended across all predefined subgroups, including those with node-negative (N0) disease, where ribociclib achieved an HR of 0.606 (95% CI: 0.372–0.986). This finding underscores the potential role of ribociclib in reducing recurrence risk even among patients traditionally considered to have a favorable prognosis.
In addition to the primary endpoint, ribociclib maintained consistent benefit across secondary measures of disease control:
- DDFS: HR = 0.709 (95% CI: 0.608–0.827)
- DRFS: HR = 0.699 (95% CI: 0.594–0.824)
Both endpoints confirm the combination’s ability to prevent distant metastases, which represent the most clinically meaningful form of relapse in early breast cancer.
Furthermore, a positive trend toward overall survival continues to emerge, with HR = 0.800 (95% CI: 0.637–1.003; nominal 1-sided P = .026), suggesting potential long-term mortality benefit that may reach statistical significance with further follow-up.
Safety
After a median follow-up of approximately two years beyond completion of ribociclib therapy, no new or unexpected safety signals were reported. The long-term safety profile remained consistent with prior analyses and with the established tolerability of ribociclib in the metastatic setting.
The 400 mg dosing strategy—lower than the 600 mg dose used in metastatic disease—was designed to balance efficacy with improved tolerability for long-term adjuvant use. The absence of cumulative or delayed toxicity supports the feasibility of prolonged CDK4/6 inhibition in early-stage management.
Long-Term Follow-Up and Analytical Considerations
With a median follow-up of 55.4 months, the NATALEE trial represents one of the most comprehensive and prolonged evaluations of adjuvant CDK4/6 inhibition in early breast cancer to date. The durability of the iDFS, DDFS, and DRFS advantages observed with ribociclib plus NSAI underscores the long-term clinical impact of early intervention.
Notably, the divergence in disease-free survival curves emerged early and continued to widen over time, suggesting that initiating ribociclib during the adjuvant phase provides durable suppression of micrometastatic disease that persists well beyond the active treatment period. The increasing absolute iDFS improvement—from 2.7% at three years to 4.5% at five years—reinforces this sustained benefit.
The positive OS trend (HR, 0.800; 95% CI: 0.637–1.003) also reflects a growing survival advantage that may reach statistical significance with further observation. Importantly, these findings were achieved despite widespread access to subsequent lines of therapy in both arms, including endocrine therapy continuation and modern targeted treatments.
From an analytical standpoint, the 5-year data confirm the robustness of the NATALEE design, which integrated both anatomical and biological risk features, enabling the inclusion of a broader early breast cancer population—particularly high-risk node-negative patients, who showed a meaningful reduction in recurrence risk (HR, 0.606).
With all patients now off ribociclib and several years of post-treatment observation completed, no new or delayed safety signals have emerged, affirming the long-term tolerability of the 400 mg dosing regimen. Together, these results provide a comprehensive view of the sustained efficacy and safety of adjuvant ribociclib plus endocrine therapy, supporting its role as a cornerstone in the evolving landscape of early HR+/HER2− breast cancer management.
The NATALEE trial thus establishes ribociclib as a viable, well-tolerated adjuvant option capable of addressing both node-positive and biologically high-risk node-negative populations—broadening the reach of precision-based therapy in early breast cancer management.
Read Full Abstract on ESMO Congress 2025 Website
You Can Watch More on OncoDaily Youtube TV
Written by Armen Gevorgyan, MD