This phase II multicenter trial explored whether metastatic colorectal cancers classified as MSI-like by gene expression—not just those confirmed MSI by PCR/IHC—could benefit from immunotherapy when combined with anti-angiogenic treatment. The idea was based on the hypothesis that blocking VEGF might improve immune infiltration and allow checkpoint inhibitors to work even in microsatellite-stable (MSS) tumors, which are typically resistant.
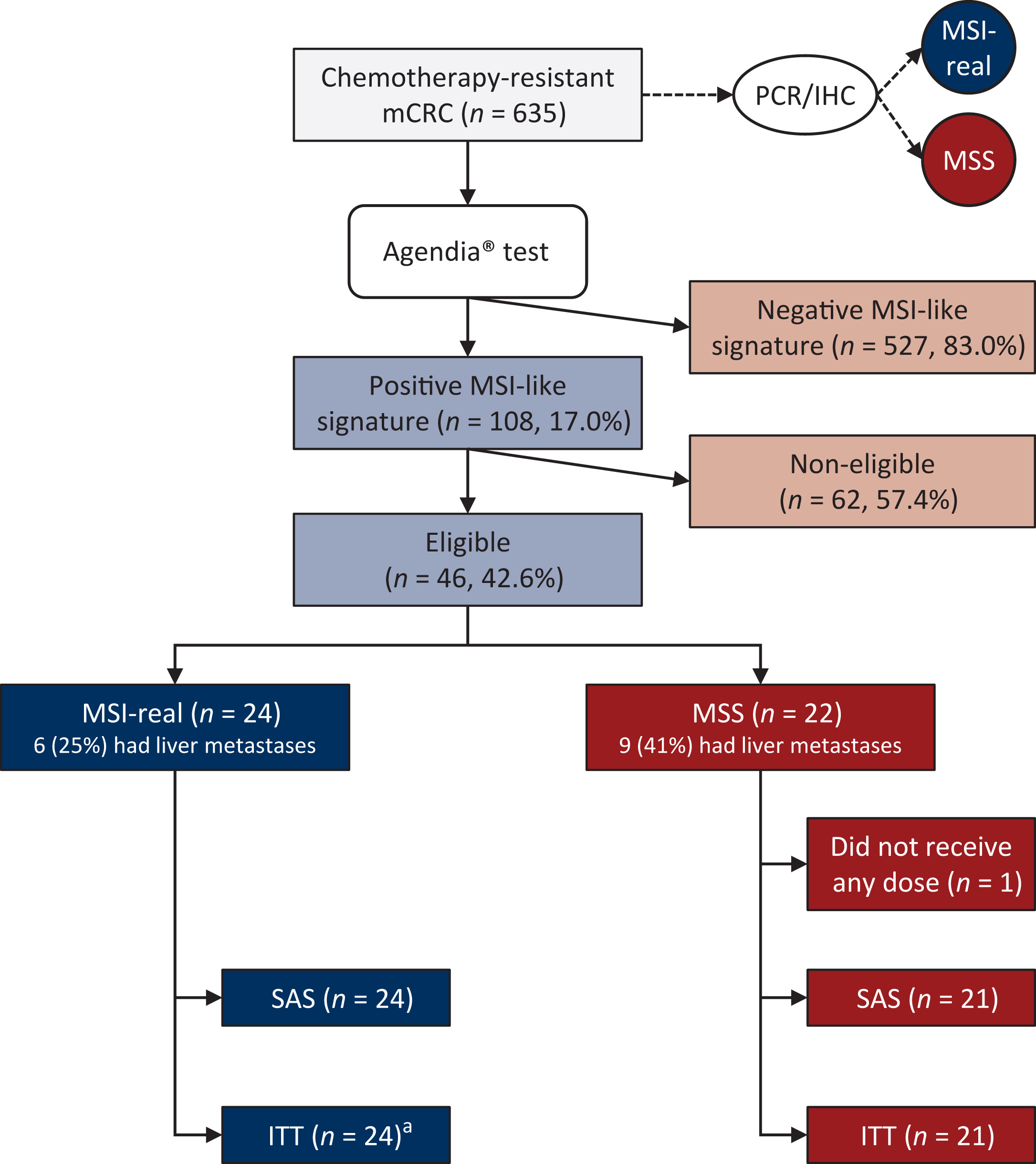
A total of 45 patients with chemotherapy-refractory metastatic CRC were enrolled across seven European centers. All had tumors labeled “MSI-like” by genomic signature, yet conventional testing revealed that 24 were true MSI and 21 were MSS. Every participant received atezolizumab (1200 mg) plus bevacizumab (7.5 mg/kg) every 3 weeks, continued until disease progression or unacceptable toxicity.
How Well Did Treatment Work?
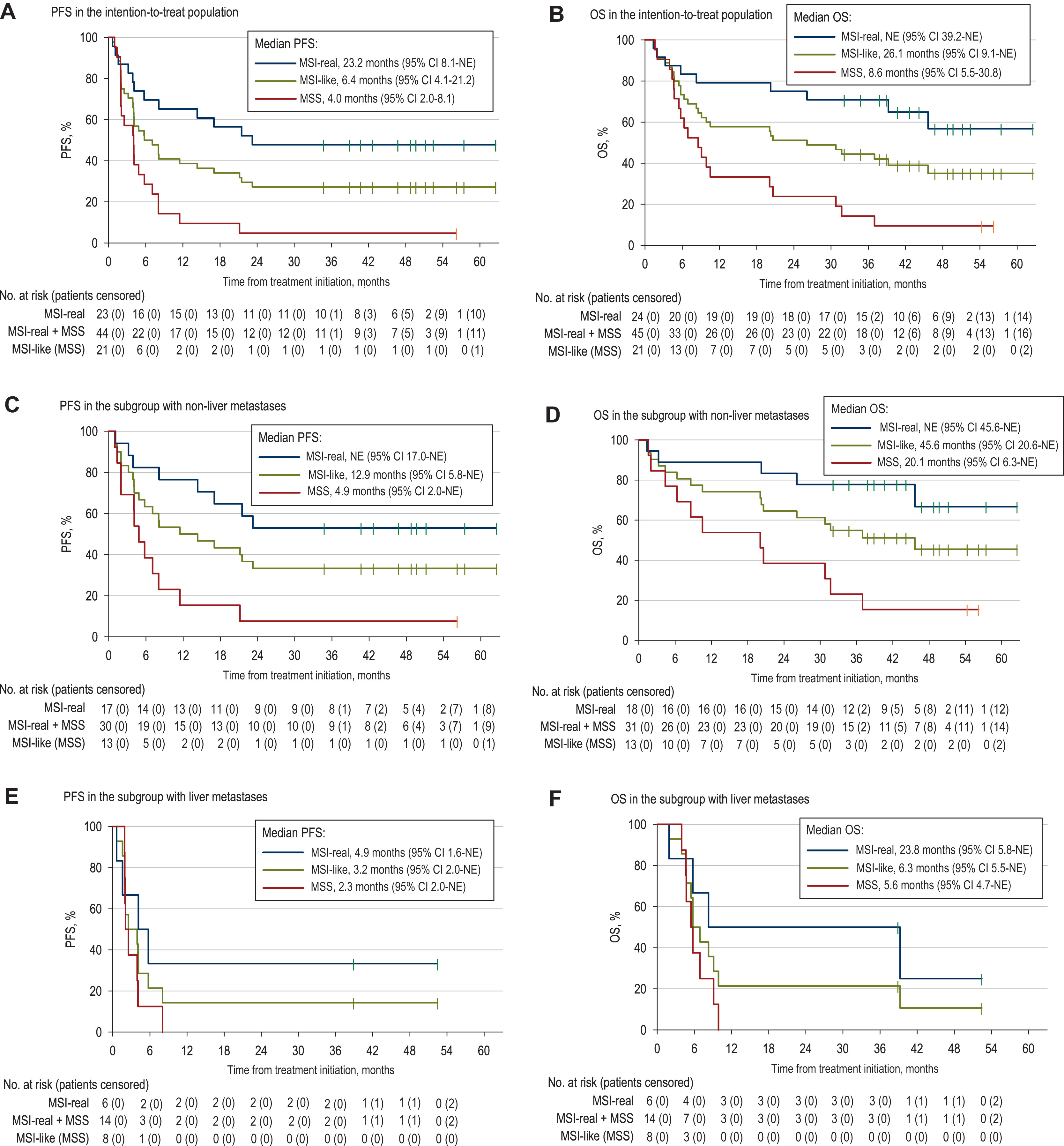
Across the entire MSI-like population, the objective response rate was 38.6%, but outcomes differed sharply by molecular status:
- MSI tumors responded strongly, with an ORR of 65.2%, long progression-free survival (median 23.2 months), and longer overall survival. Responses were often deep and durable.
- MSS tumors showed minimal benefit, with an ORR of only 9.5% and a median PFS of about 4 months.
- A notable exception: MSS patients without liver metastases showed some sensitivity (ORR ~15%), suggesting that the liver microenvironment may be a barrier to immune activity.
Overall median PFS for all MSI-like colorectal cancer patients was 6.4 months, but when separated, results clearly favored MSI tumors. Durability also followed this pattern—MSI patients maintained responses far longer than MSS patients.
Safety and Tolerability
Nearly all patients experienced treatment-related side effects, which is expected for immune checkpoint and anti-VEGF therapy combinations.
Most common toxicities were:
- Fatigue
- Hypertension
- Diarrhea
- Abdominal pain
About half (48.9%) had grade 3 or higher events.
Severe toxicity was more often linked to bevacizumab than atezolizumab. Two deaths occurred—one from colonic hemorrhage likely related to bevacizumab, and one unrelated.
There were no unexpected safety signals, and overall the combination was considered manageable.
What Do These Results Mean?
This study confirms that:
- MSI metastatic CRC responds very well to immunotherapy, and VEGF inhibition may further enhance outcomes.
- MSS tumors remain largely resistant, even if labeled MSI-like by gene expression.
- Absence of liver metastasis may identify a small MSS subgroup that could benefit, though evidence remains limited.
Importantly, the MSI-like genomic classifier did not successfully expand immunotherapy eligibility beyond standard MSI definitions, meaning traditional PCR/IHC testing still remains the most clinically reliable biomarker.
Conclusion
Atezolizumab + bevacizumab demonstrated strong antitumor activity in MSI-confirmed mCRC, validating the dual-blockade approach. However, the MSI-like gene expression signature did not expand immunotherapy eligibility to MSS disease, and benefit in MSS patients was limited and predominantly seen in those without liver metastases.
These results emphasize the need for refined predictive biomarkers and a deeper understanding of immune evasion in MSS CRC, particularly the impact of the liver microenvironment.
You can read all article here


