The monarchE trial at ESMO 2025 (NCT03155997) has achieved a key milestone in the management of hormone receptor–positive (HR+), HER2-negative, node-positive, high-risk early breast cancer (EBC). Two years of adjuvant abemaciclib combined with endocrine therapy (ET) demonstrated a statistically significant and clinically meaningful overall survival (OS) improvement over ET alone, confirming the long-term benefit of CDK4/6 inhibition in the adjuvant setting.
Previous analyses from the monarchE trial at ESMO 2025 also showed durable gains in invasive disease-free survival (IDFS) and distant relapse-free survival (DRFS). The current report presents the primary OS analysis along with updated IDFS and DRFS results after a median follow-up of 6.3 years, reinforcing abemaciclib’s role as an effective adjuvant therapy for high-risk HR+, HER2− early breast cancer.
Methods
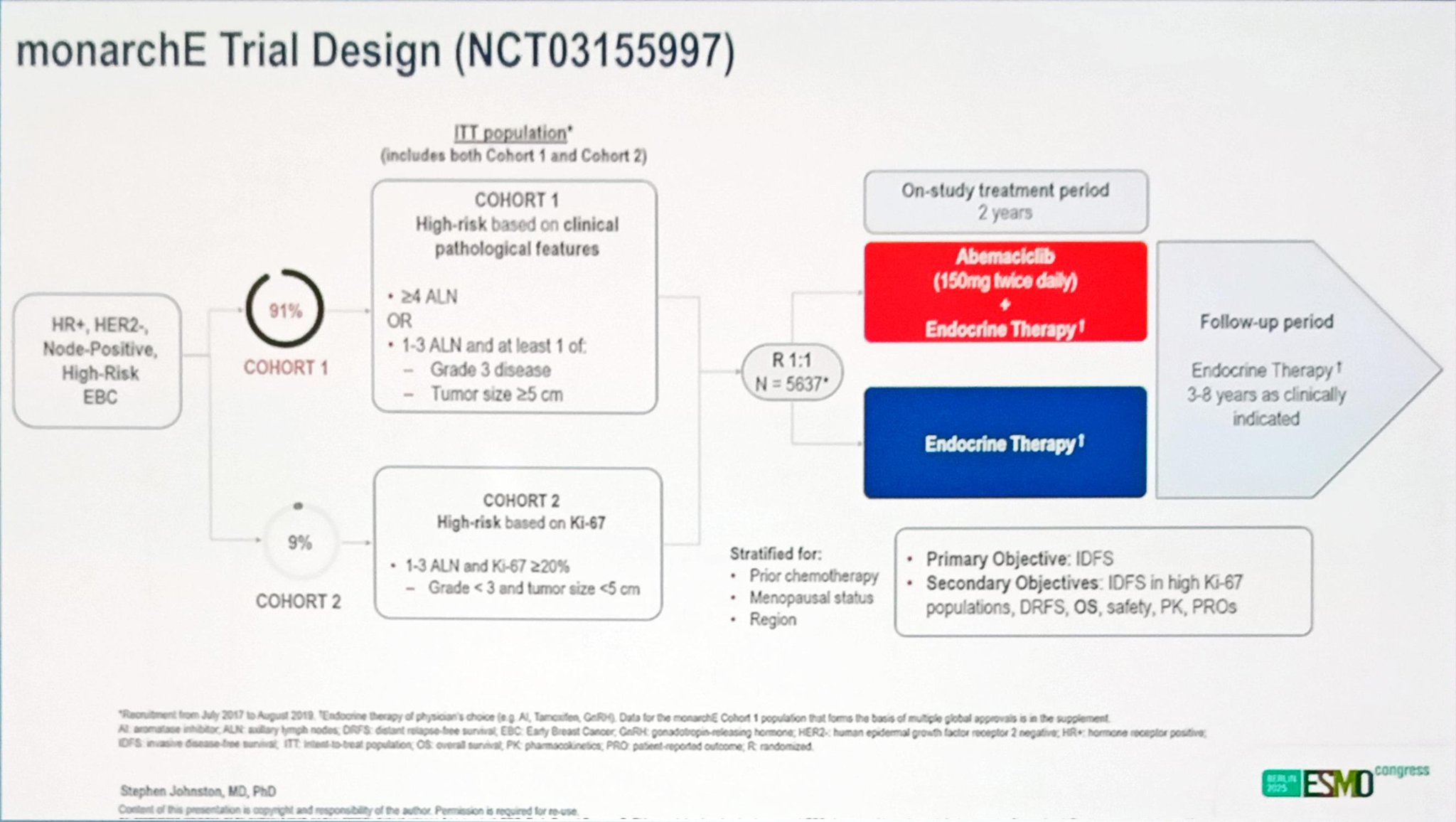
monarchE is an open-label, randomized Phase 3 study enrolling patients with HR+, HER2−, high-risk early breast cancer. Participants were randomized 1:1 to receive standard ET for at least five years with or without abemaciclib for the first two years.
High-risk disease was defined as either:
- ≥4 positive axillary lymph nodes (ALN), or
- 1–3 positive ALN with Grade 3 histology and/or tumor size ≥5 cm (Cohort 1).
A second cohort (Cohort 2) included patients with 1–3 positive ALN and centrally confirmed Ki67 ≥20%.
The intent-to-treat (ITT) population included 5120 patients in Cohort 1 and 517 in Cohort 2. The primary OS analysis was preplanned to occur after approximately 650 deaths in the ITT population.
Results
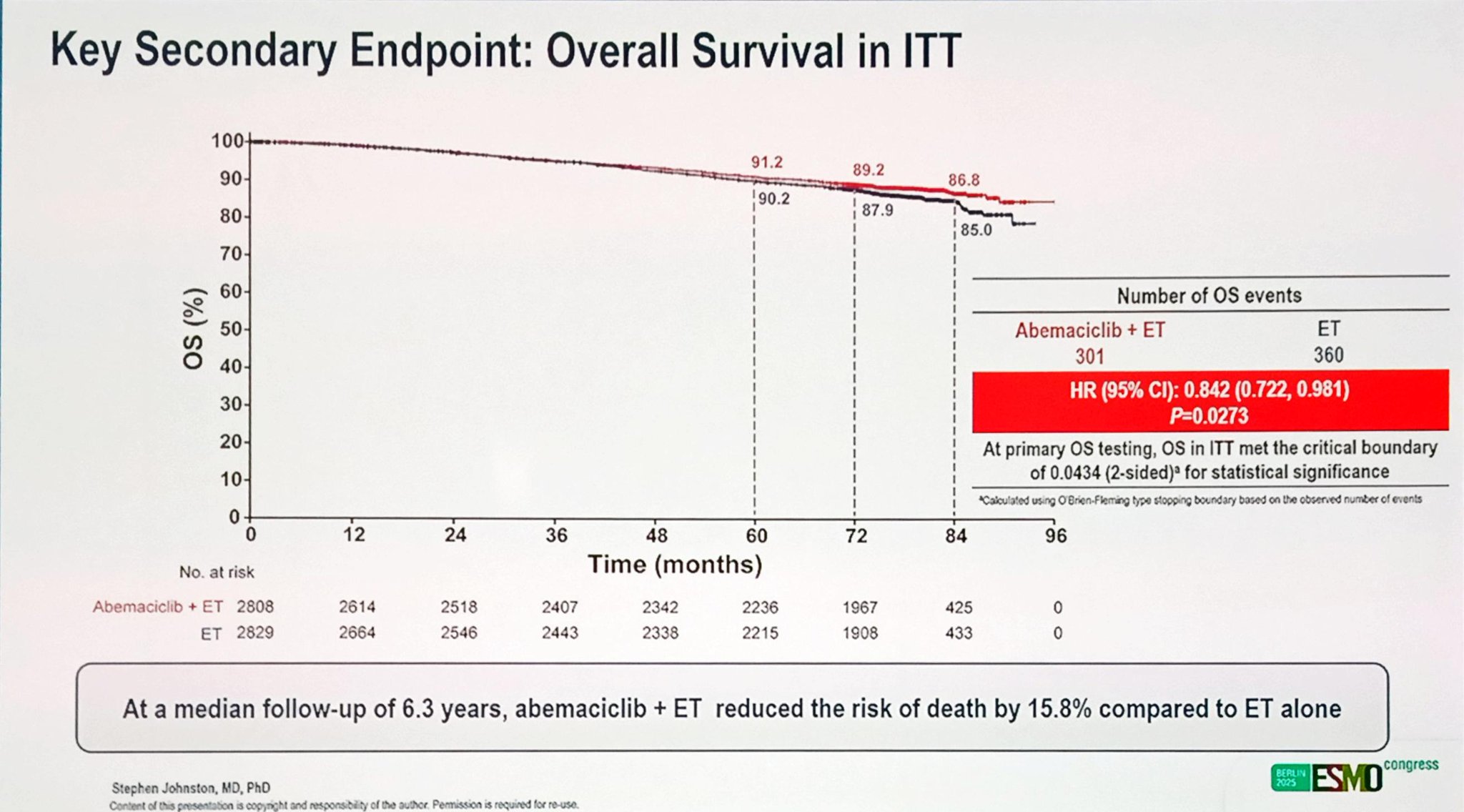
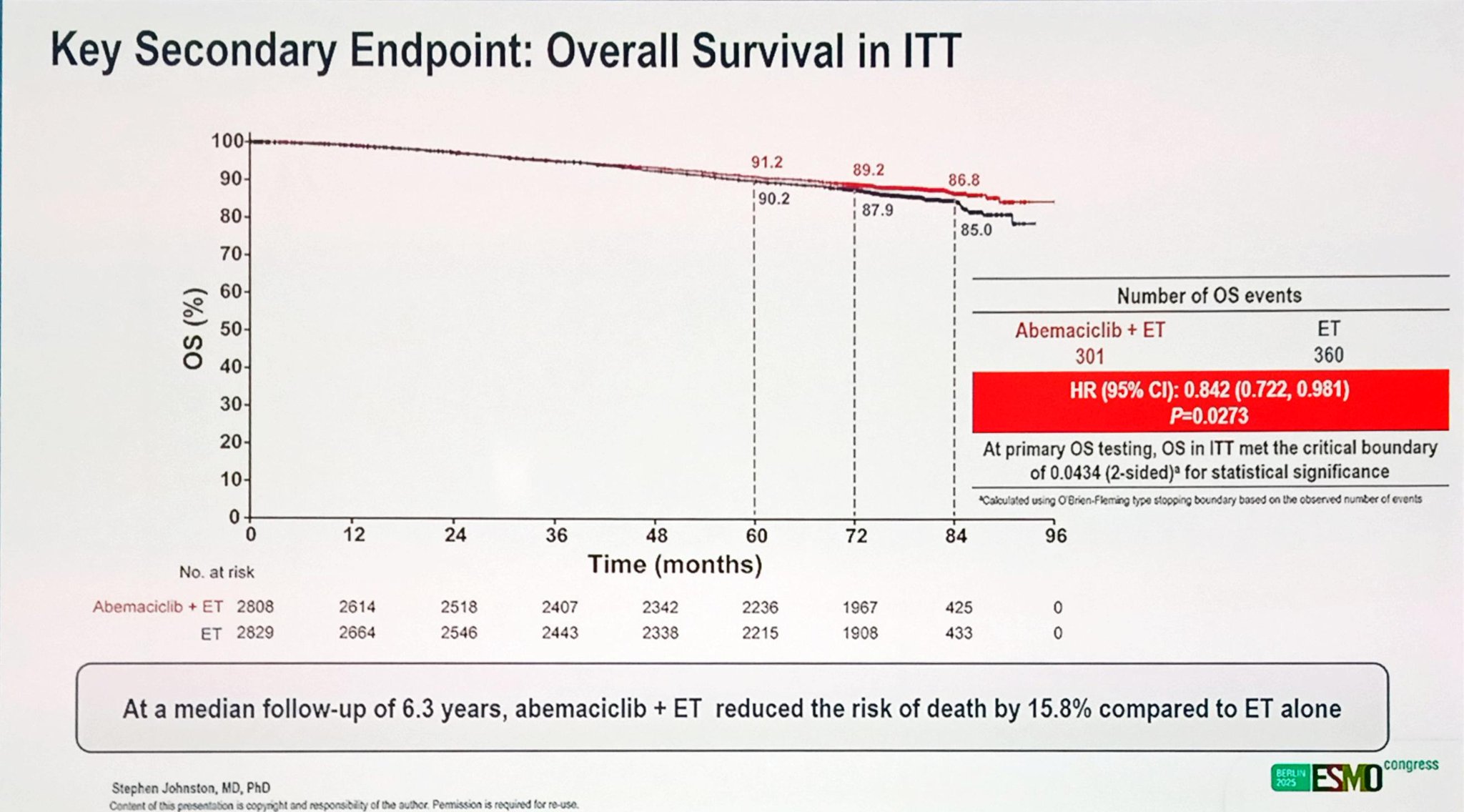
At the time of analysis, after a median follow-up of 6.3 years, 301 deaths were reported in the abemaciclib + ET arm and 360 deaths in the ET-alone arm. The addition of abemaciclib to ET reduced the risk of death by 15.8% compared to ET alone, corresponding to a hazard ratio (HR) of 0.84 (95% CI, 0.72–0.98; P = 0.027). This met the prespecified boundary for statistical significance.
The OS benefit was consistent across all prespecified subgroups.
Long-term analysis confirmed sustained benefit in disease control. The IDFS HR was 0.73 (95% CI, 0.66–0.82) and the DRFS HR was 0.75 (95% CI, 0.66–0.84), both favoring the abemaciclib + ET arm.
At 7 years,
- IDFS was 77.4% with abemaciclib + ET vs 70.9% with ET alone — an absolute improvement of 6.5%, and
- DRFS was 80.0% vs 74.9%, showing an absolute benefit of 5.1%.
A higher proportion of patients in the ET-alone group (52%) received subsequent CDK4/6 inhibitors in the metastatic setting compared with 34% in the abemaciclib arm, yet the OS advantage with adjuvant abemaciclib persisted.
Outcomes in Cohort 1 mirrored the overall ITT population, confirming consistency across high-risk subgroups. Long-term safety results showed no evidence of delayed or cumulative toxicities, reinforcing the tolerability of the two-year abemaciclib regimen.
Long-Term Follow-Up and Analytical Considerations
At ESMO 2025, investigators emphasized that the monarchE trial represents one of the longest-running CDK4/6 inhibitor studies in the adjuvant setting, with data now extending beyond seven years from initial enrollment. This extended follow-up allowed for comprehensive evaluation not only of survival outcomes but also of event dynamics over time.
The updated analyses demonstrated that the divergence between treatment arms emerged early—within the first two years of therapy—and continued to widen through year seven, supporting the hypothesis that early CDK4/6 inhibition provides durable suppression of micrometastatic disease. Importantly, the durability of benefit persisted long after discontinuation of abemaciclib, reinforcing the biological rationale for finite, time-limited treatment in early breast cancer.
Investigators also noted that survival results were obtained in the context of modern post-progression treatment, with widespread access to subsequent lines of therapy, including CDK4/6 inhibitors in the metastatic setting. Despite this, the OS improvement remained statistically significant, underscoring the strength and clinical relevance of early intervention.
Together, these findings highlight the sustained impact of adjuvant abemaciclib on long-term patient outcomes and further validate CDK4/6 inhibition as a cornerstone strategy in the treatment of high-risk HR+, HER2− early breast cancer.
Read Full Abstract on ESMO Congress 2025 Website
You Can Watch More on OncoDaily Youtube TV
Written by Armen Gevorgyan, MD


