The phase III PIVOTAL trial is the first study to demonstrate a significant clinical benefit of neoadjuvant intralesional immunotherapy in resectable stage III melanoma. The investigational therapy, daromun, combines two targeted immunocytokines—L19IL2 (interleukin-2) and L19TNF (tumor necrosis factor)—designed to bind selectively to the extradomain B (EDB) of fibronectin, a tumor-associated neoangiogenic marker. This dual construct localizes immune activation within the tumor microenvironment, aiming to enhance systemic antitumor immunity while minimizing systemic toxicity.
What Is Daromun?
Daromun is an innovative intralesional targeted immunocytokine therapy designed to stimulate a localized and systemic immune response against melanoma. It is a fixed combination of two antibody–cytokine fusion proteins:
- L19IL2 – a fusion of the L19 antibody fragment (which specifically binds to the extra domain B (EDB) of fibronectin, a marker of tumor-associated neovasculature) and interleukin-2 (IL-2), a cytokine known to activate T cells and natural killer (NK) cells.
- L19TNF – a fusion of the same L19 antibody fragment with tumor necrosis factor-alpha (TNF-α), a cytokine that can induce vascular disruption and enhance immune cell infiltration in tumors.
By combining these two immune-stimulating agents with tumor-targeting antibodies, daromun delivers cytokines directly to the tumor microenvironment (TME). This design amplifies local immune activation while minimizing systemic exposure and toxicity.
Study Design
The PIVOTAL trial (NCT02938299) was an open-label, randomized, multicenter phase III study conducted across 22 sites in Germany, Italy, France, and Poland.
A total of 246 patients with clinically detectable stage IIIB/C melanoma were randomized 1:1 to receive either:
- Neoadjuvant daromun (13 million IU L19IL2 + 400 µg L19TNF, intralesional weekly ×4 weeks) followed by surgery, or
- Upfront surgery without prior intralesional therapy.
Both arms allowed subsequent adjuvant systemic therapy at the discretion of treating physicians. The primary endpointwas recurrence-free survival (RFS) assessed by blinded independent central review (BICR), while secondary endpoints included distant metastasis-free survival (DMFS), overall survival (OS), and pathologic response rates.
Patient Characteristics
The population represented a high-risk cohort, with 74% of patients having undergone two or more prior surgeries and 35% previously treated with systemic therapy, including immune checkpoint inhibitors. The median age was 64 years, and 70% had stage IIIC disease per AJCC 8th edition. About 40% presented with in-transit metastases, known to predict poorer outcomes.
Results
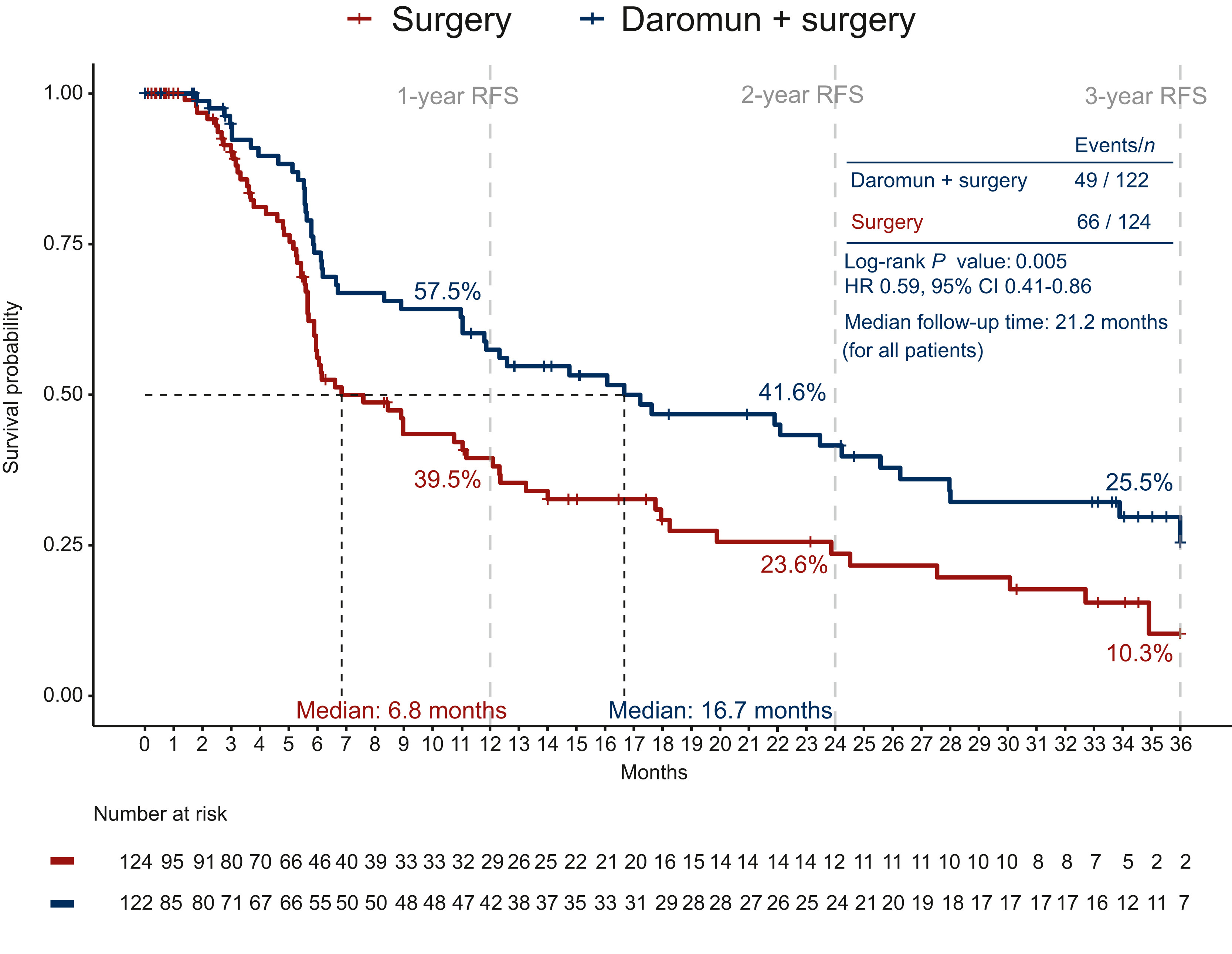
At a median follow-up of 21 months, the neoadjuvant daromun arm showed superior recurrence-free survivalcompared to upfront surgery:
- Median RFS: 16.7 months vs. 6.8 months
- 2-year RFS: 41.6% vs. 23.6%
- Hazard ratio (HR): 0.59; 95% CI: 0.41–0.86; p = 0.005
The risk of distant metastasis was also reduced by 40% (HR 0.60; 95% CI: 0.37–0.95; p = 0.029).
A pathologic complete response (pCR) was achieved in 21% of patients who underwent surgery after daromun therapy, supporting its potent local and systemic immune activity.
The benefit was consistent across prespecified subgroups, regardless of age, gender, BRAF mutation status, or prior systemic therapy.
Safety
Neoadjuvant daromun was well tolerated, with treatment-related adverse events (TRAEs) mainly limited to local reactions:
- Grade ≥3 TRAEs: 26% vs. 6.7% (control)
- Most common AEs: injection site reactions (67%), pyrexia (48%), chills (48%), nausea (22%)
- Immune-related AEs: 2.5%, all mild (grade 1), transient, and manageable
- No treatment-related deaths occurred.
The safety profile contrasts favorably with systemic neoadjuvant immunotherapy, which typically shows grade ≥3 AEs in up to 47% of patients.

You Can Read All Article Here


