Malignant pleural mesothelioma (MPM) remains a challenging cancer with limited treatment options, but emerging gold-based immunotherapy offers new hope. This study investigates the potential of Au(III)–DTC complexes as immunogenic cell death (ICD) inducers, transforming “cold” tumors into “hot” tumors that are more responsive to immune checkpoint inhibitors like nivolumab and ipilimumab. By triggering key ICD markers such as calreticulin (CRT) exposure, ATP release, and HMGB1 secretion, these novel metal-based compounds enhance antitumor immune responses without systemic toxicity. Preclinical models demonstrated that gold complexes 1C and 2G significantly inhibited tumor growth and promoted long-term tumor-free survival, surpassing the efficacy of standard cisplatin-pemetrexed chemotherapy. With their favorable safety profile and durable immune activation, these next-generation gold-based cancer therapies could revolutionize mesothelioma treatment and reshape the future of metal-based immunotherapies.
Title: Leveraging Immunogenic Cell Death to Enhance the Immune Response against Malignant Pleural Mesothelioma Tumors
Authors: Meng Rui Chang, Egor M. Matnurov, Chengnan, Jemma Arakelyan, Ho-Jung Choe, Vladimir Kushnarev, Jian Yu Yap, Xiu Xuan Soo, Mun Juinn Chow, Walter Berger, Wee Han Ang, Maria V. Babak
Published in JACS, Feb 2025
Background
Malignant pleural mesothelioma (MPM) is a highly aggressive cancer primarily caused by asbestos exposure. Standard treatment options, such as platinum-based chemotherapy (cisplatin-pemetrexed), have demonstrated limited efficacy, with median survival rates of approximately 12–18 months. Immunotherapy, particularly immune checkpoint inhibitors like nivolumab and ipilimumab, has improved survival in some patients, but many cases remain resistant.
Immunogenic cell death (ICD) is a form of regulated cell death that activates an adaptive immune response against tumor cells. Unlike other types of cell death (such as apoptosis or necrosis), ICD releases specific molecules called damage-associated molecular patterns (DAMPs), such as calreticulin (CRT) exposure, ATP release, and HMGB1 secretion, which alert and activate the immune system.
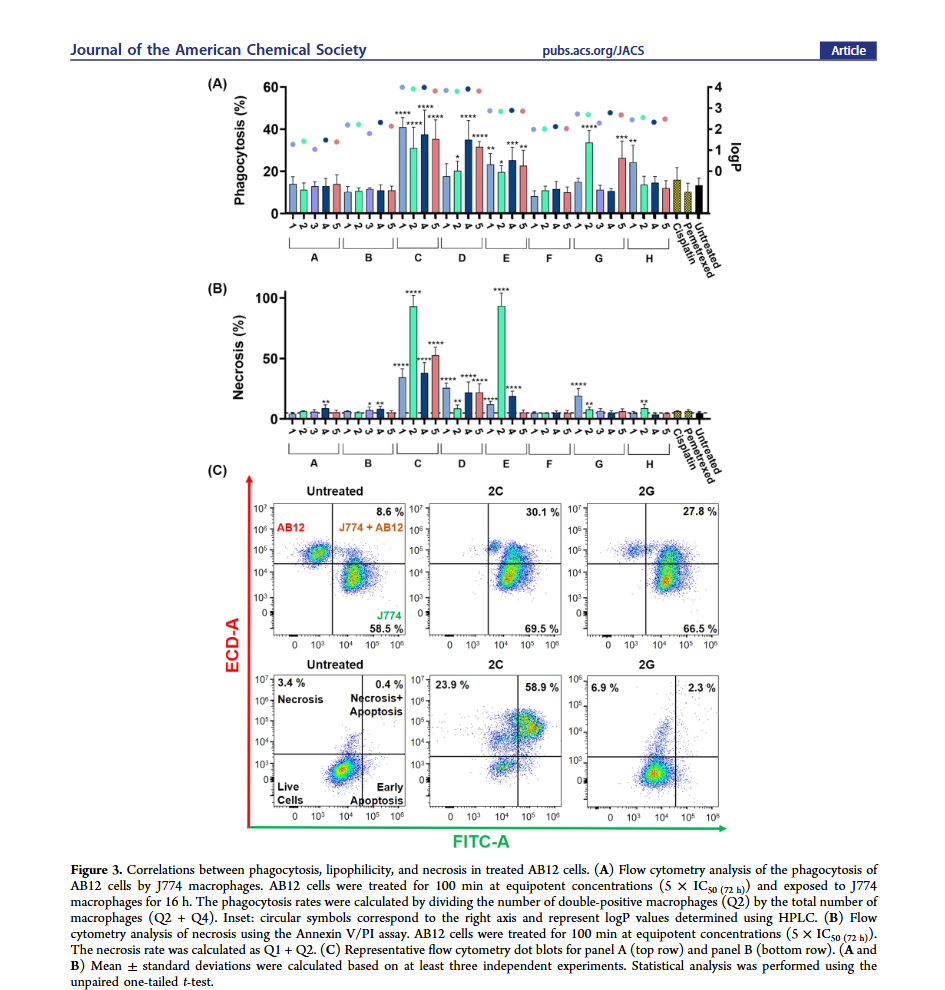
Immunogenic cell death (ICD) has emerged as a promising approach in cancer therapy, as it can stimulate an antitumor immune response by exposing tumor-associated antigens. ICD plays a crucial role in cancer immunotherapy by converting “cold” tumors (those with little immune infiltration) into “hot” tumors that are more likely to respond to immune-based treatments. Metal-based compounds, especially gold (Au) and platinum (Pt) complexes, have shown potential as ICD inducers, but their mechanisms remain poorly understood. This study aims to investigate the ability of cyclometalated Au(III)–DTC complexes to induce ICD in vitro and in vivo, with a particular focus on their potential as therapeutic agents for MPM.
Study Design and Methods
To explore the ICD-inducing properties of Au(III) complexes, the researchers synthesized and characterized 35 different Au(III)–DTC compounds. These complexes were selected based on variations in their cyclometalated scaffolds and ligand structures, which influence their lipophilicity, electronic properties, and ability to target the endoplasmic reticulum (ER).
In vitro ICD screening
- The complexes were tested against immunologically “cold” mesothelioma cell lines to assess their ability to trigger phagocytosis by immune cells.
- The researchers measured key ICD markers, including CRT surface exposure, ATP release, and HMGB1 secretion, which are necessary for an effective immune response.
In vivo efficacy testing for malignant pleural mesothelioma
- The most promising compounds, 1C and 2G, were selected for further evaluation in an AB12 mesothelioma allograft model in immunocompetent BALB/c mice.
- Mice were treated with 1C (1.5 mg/kg) or 2G (3.5 mg/kg) at their maximum soluble doses, using a 4% DMSO/Cremophor EL in PBS formulation.
- Tumor growth inhibition, immune response activation, and potential toxicity were evaluated.
- Flow cytometry and immunohistochemistry (IHC) were performed to analyze macrophage populations and the expression of key immune markers in tumor tissues.
- Long-term immunity was assessed by vaccinating mice with tumor cells treated with Au(III) complexes and monitoring tumor-free survival.
Results
Both 1C and 2G exhibited potent antitumor effects in vivo, significantly reducing tumor growth compared to vehicle-treated controls. Importantly, neither complex caused weight loss or systemic toxicity, as indicated by normal histological examinations of liver and kidney tissues.
Histological and Immunological Analysis:
- Necrosis and CRT Exposure:
- 1C induced significant necrosis in tumors, affecting up to 80% of the tissue.
- 2G-treated tumors exhibited minimal necrosis (0%–10%).
- CRT expression was notably elevated in non-necrotic regions of 1C-treated tumors, suggesting an ICD-driven immune response.
- Macrophage Analysis:
- Flow cytometry and IHC staining of tumor tissues showed no significant differences in overall macrophage populations between treatment groups.
- IBA1 staining, used as a macrophage activation marker, showed no significant changes across groups, confirming that neither 1C nor 2G depleted macrophages.
- Long-Term Immunogenicity:
- Mice vaccinated with 2G-treated tumor cells demonstrated prolonged tumor-free survival.
- 7 out of 8 mice remained tumor-free for 164 days (5.5 months), and 4 out of 8 remained tumor-free for 210 days (7 months).
- This extended tumor-free period is clinically significant, as the CheckMate 743 trial demonstrated that immunotherapy extended median survival in MPM patients by only 4 months compared to chemotherapy.
- Organ-Specific Effects:
- CRT expression was not elevated in healthy liver and kidney tissues, suggesting that 1C selectively activated immune responses in tumor tissues without affecting normal organs.
Key Takeaway Messages
- Gold(III) Complexes as ICD Inducers:
- Both 1C and 2G effectively induced ICD, highlighting their potential as novel immunotherapy agents for MPM.
- Different Mechanisms of Action:
- 1C induced high levels of necrosis and CRT exposure, while 2G promoted phagocytosis without necrosis, suggesting different pathways of ICD activation.
- Long-Lasting Antitumor Immunity:
- 2G-treated tumors provided durable immune protection, with long-term tumor-free survival observed in immunocompetent mice.
- Favorable Safety Profile:
- Neither 1C nor 2G caused systemic toxicity or depleted macrophages, supporting their potential for clinical application.
- Superiority Over Conventional Chemotherapy:
- Au(III) complexes demonstrated better tumor control than the standard cisplatin-pemetrexed regimen in preclinical models.
Conclusion
This study provides crucial insights into the mechanisms underlying ICD induction by Au(III)–DTC complexes. The findings indicate that certain gold complexes can effectively trigger an immune response against MPM, leading to durable tumor suppression. Notably, 2G demonstrated superior long-term immunogenicity without significant necrosis, making it a strong candidate for further development.
While the study supports the clinical potential of Au(III) complexes, further research is needed to identify their precise biomolecular targets. Future studies should focus on optimizing these compounds to enhance their ICD-inducing properties while minimizing potential immunosuppressive effects. Bio-orthogonal or photoactivated Au-based prodrug strategies may help target tumors more selectively, reducing risks to the systemic immune system.
These findings open new avenues for the development of metal-based immunotherapies, offering a promising alternative to existing treatments for MPM. By refining the structure-activity relationships of ICD-inducing compounds, researchers can move closer to the rational design of next-generation cancer immunotherapies.
You can read the full article here
Written by Sona Karamyan, MD


