KEYNOTE-689 trial,published in The New England Journal of Medicine on June 18, 2025, offers a new hope for patients with resectable, locally advanced head and neck squamous-cell carcinoma (HNSCC). Presented by Dr. Ravindra Uppaluri and colleagues, the study evaluated whether adding perioperative pembrolizumab—administered both before and after surgery—to standard treatment could improve event-free survival (EFS). Building on previous evidence from smaller studies and other tumor types, KEYNOTE-689 provides the first large-scale evidence supporting the integration of immunotherapy into curative-intent treatment for head and neck cancer.
Tilte: Neoadjuvant and Adjuvant Pembrolizumab in Locally Advanced Head and Neck Cancer
Authors: Ravindra Uppaluri, M.D., Ph.D., Robert I. Haddad, M.D., Yungan Tao, M.D., Ph.D., Christophe Le Tourneau, M.D., Ph.D. , Nancy Y. Lee, M.D., William Westra, M.D., Rebecca Chernock, M.D., Makoto Tahara, M.D., Ph.D., Kevin J. Harrington, M.B., B.S., Ph.D., Arkadiy L. Klochikhin, M.D., Irene Braña, M.D., Ph.D., Gustavo Vasconcelos Alves, M.D., Brett G. M. Hughes, M.D., Marc Oliva, M.D., Ph.D., Iane Pinto Figueiredo Lima, M.D., Tsutomu Ueda, M.D., Ph.D., Tomasz Rutkowski, M.D., Ph.D., Ursula Schroeder, M.D., Paul-Stefan Mauz, M.D., Thorsten Fuereder, M.D., Simon Laban, M.D., Nobuhiko Oridate, M.D., Ph.D., Aron Popovtzer, M.D., Nicolas Mach, M.D., Yevhen Korobko, M.D., Ph.D., Diogo Alpuim Costa, M.D., Anupama Hooda-Nehra, M.D.27,28, Cristina P. Rodriguez, M.D.29, R. Bryan Bell, M.D., Cole Manschot, Ph.D., Kimberly Benjamin, M.D., Burak Gumuscu, M.D., Ph.D., and Douglas Adkins, M.D., for the KEYNOTE-689 Investigators.
Published in NEJM , June 2025
Background
Locally advanced head and neck squamous-cell carcinoma (HNSCC) is a challenging disease with stagnant therapeutic progress over two decades. Despite surgery followed by radiotherapy (± cisplatin) being standard, about one-third relapse within one year and fewer than half survive to 5 years Pembrolizumab (anti‑PD‑1) has shown benefit in metastatic/recurrent HNSCC and phase 2 perioperative trials hinted at improved disease control when added to surgery and adjuvant therapy KEYNOTE‑689 (phase 3) evaluated whether adding neoadjuvant and adjuvant pembrolizumab to standard care improves event-free survival (EFS) in resectable, locally advanced HNSCC.
Methods and Study Design of Keynote-689 Trial
The KEYNOTE-689 study was an international, open-label, randomized phase 3 clinical trial conducted across 192 centers. It enrolled adults with newly diagnosed, non-metastatic, resectable stage III or IVA head and neck squamous-cell carcinoma, including cancers of the oropharynx, larynx, hypopharynx, or oral cavity.
Eligible participants had an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1 and were required to undergo tumor testing for PD-L1 expression and HPV status. A total of 714 participants were randomly assigned in a 1:1 ratio to receive either standard of care alone—consisting of surgery followed by adjuvant radiotherapy with or without cisplatin—or standard of care combined with neoadjuvant and adjuvant pembrolizumab. Randomization was stratified according to primary tumor site, disease stage, and PD-L1 tumor proportion score (≥50% versus <50%).
- Pembrolizumab arm- 2 cycles neoadjuvant (200 mg IV q3w), then surgery within 6 weeks.Adjuvant pembrolizumab (200 mg q3w): 3 cycles concurrent with RT/CRT, then 12 additional cycles.
- Control arm: Standard surgery within 4 weeks and adjuvant therapy determined by pathology: Low-risk: Radiotherapy 60 GyHigh-risk (positive margins or extranodal extension): Chemoradiotherapy 66 Gy + cisplatin.
Treatment continued until progression, unacceptable toxicity, death, or consent withdrawal
Endpoints
- Primary: EFS in (1) PD‑L1 CPS ≥10, (2) CPS ≥1, and (3) total population.
- Secondary: Major pathologic response (≤10% viable tumor), pathological complete response, overall survival (OS), and safety.
Hierarchical testing with Lan–DeMets O’Brien–Fleming boundaries; interim analysis at 207 CPS‑10 events (~38 months median follow-up)
Results of Keynote 689 trial
At the data cutoff of July 25, 2024, a total of 714 patients had undergone randomization—363 to the pembrolizumab group and 351 to the control group. The baseline characteristics were well balanced across both arms, including the proportions of participants with PD-L1 combined positive scores (CPS) of ≥10 or ≥1, which were consistent with expectations based on previous studies.
Surgery was completed in approximately 88% of participants in both groups, indicating that neoadjuvant pembrolizumab did not compromise the feasibility of surgical intervention. Postoperative therapy was initiated in roughly three-quarters of patients across both arms, though the proportion receiving adjuvant cisplatin was lower in the pembrolizumab group. With a median follow-up duration of 38.3 months, the first prespecified interim analysis provided sufficient data to assess the primary endpoint of event-free survival and to evaluate key secondary endpoints including pathological response rates and overall survival trends.
Event‑Free Survival
- CPS ≥10- 3‑year EFS: 59.8% vs. 45.9% (HR 0.66; 95% CI 0.49‑0.88; P=0.004)
- CPS ≥1- 58.2% vs. 44.9% (HR 0.70; 95% CI 0.55‑0.89; P=0.003).
- Total cohort- 57.6% vs. 46.4% (HR 0.73; 95% CI 0.58‑0.92; P=0.008)
Pathologic Response
- Major pathological response- CPS ≥10: +13.7 pp (P<0.001); CPS ≥1: +9.8 pp; total: +9.3 pp (all P<0.001)
- Pathologic complete response: Approx. 3 pp benefit across groups .
Overall Survival (Interim)
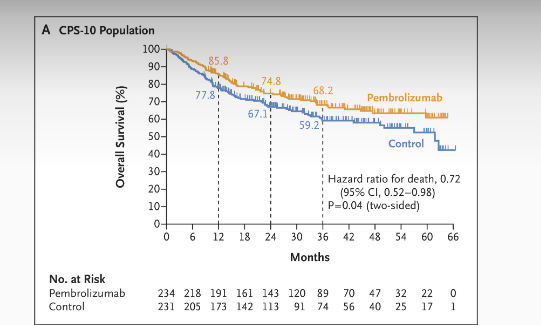
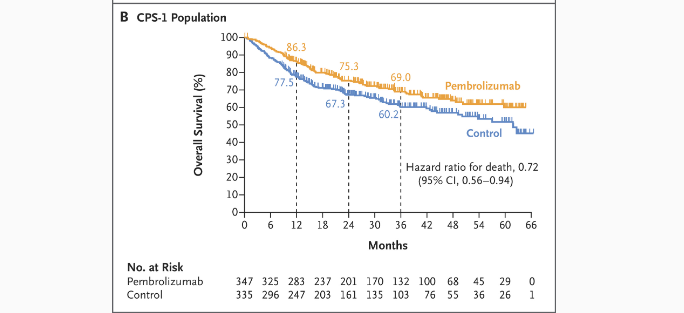
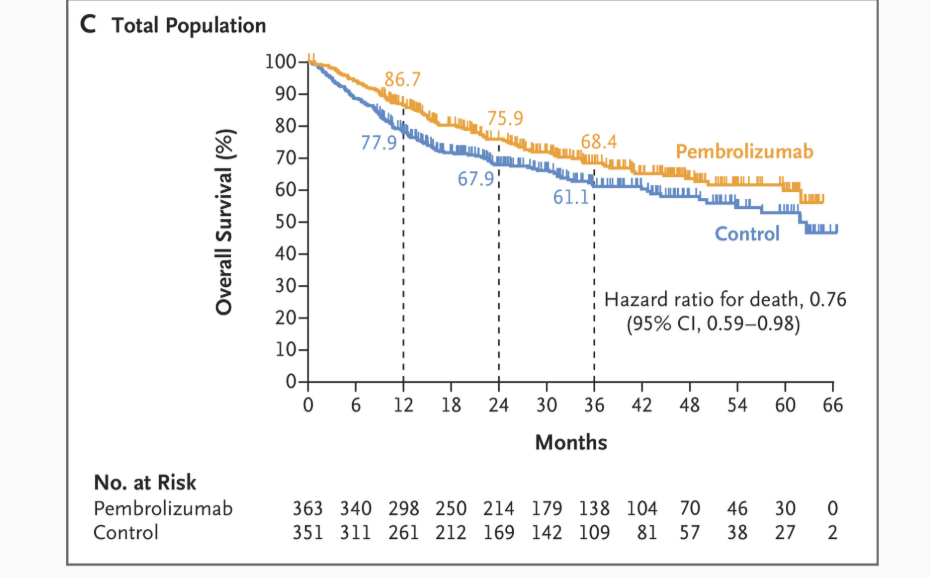
- CPS ≥10: 3‑year OS 68.2% vs. 59.2% (HR 0.72; 95% CI 0.52‑0.98; P=0.04) — not formally tested due to statistical hierarchy.
- CPS ≥1: 69.0% vs. 60.2% (HR 0.72; 95% CI 0.56‑0.94).
- Total: 68.4% vs. 61.1% (HR 0.76; 95% CI 0.59‑0.98) .
Safety
- Any‑grade TRAEs: 81% in both arms; ≥Grade 3: 44.6% vs. 42.9%.
- TRAE‑related deaths: 1.1% (renal failure, COVID‑19 pneumonia, pneumonitis, unknown) vs. 0.3%.
- Serious TRAEs: 19.1% vs. 10.5%; immune-mediated ≥Grade 3: 10.0% vs. 0.6%
- Most common ≥Grade 3 events: stomatitis (~12%), pneumonitis (1.9% pembrolizumab), hypothyroidism (24.7%) .
Key Findings
KEYNOTE‑689 marks a practice-changing advancement in curative-intent therapy for locally advanced, resectable HNSCC. Adding neoadjuvant and adjuvant pembrolizumab to standard surgery and adjuvant RT/CRT significantly improved 3‑year EFS (from ~46% to ~58‑60%) and triggered earlier and durable separation of Kaplan–Meier curves. Pathologic response data reinforce its neoadjuvant “priming” effect. Although final OS data are pending, trends are favorable. The regimen’s safety profile
- ~27% reduction in EFS events across all PD‑L1 subgroups (HR 0.66‑0.73).
- Major pathologic responses nearly 10% higher with pembrolizumab.
- 3‑year OS improvement suggested, but final analysis pending per hierarchy.
- No negative impact on surgical completion, despite neoadjuvant immunotherapy.
- Immune-related toxicity expected but manageable, with no novel safety signals.
Key Take‑Away Messages
- Added perioperative pembrolizumab yields substantial EFS benefit (HR ~0.7) in resectable, locally advanced HNSCC.
- Pathologic responses provide early biological evidence of efficacy.
- Early OS trends are promising, though confirmatory follow-up is needed.
- Safety profile aligns with known pembrolizumab effects (hypothyroidism, pneumonitis), reinforcing predictable management.
- Clinical milestone: first perioperative immune-checkpoint approval in HNSCC in approximately six years
You can read the full article here.
Written by Sona Karamyan, MD


