Non–small cell lung cancer (NSCLC) remains the leading cause of cancer-related mortality worldwide. In early-stage disease, complete surgical resection offers the best chance for cure. However, recurrence rates remain significant, particularly in stage II–IIIA disease.
For years, adjuvant platinum-based chemotherapy was the standard approach for appropriate stages, reducing recurrence risk but leaving substantial residual relapse rates. This unmet need led to the evaluation of immune checkpoint inhibitors in the adjuvant setting, with the goal of improving disease-free survival (DFS) and potentially overall survival (OS).
Adjuvant Pembrolizumab: KEYNOTE-091 (PEARLS)
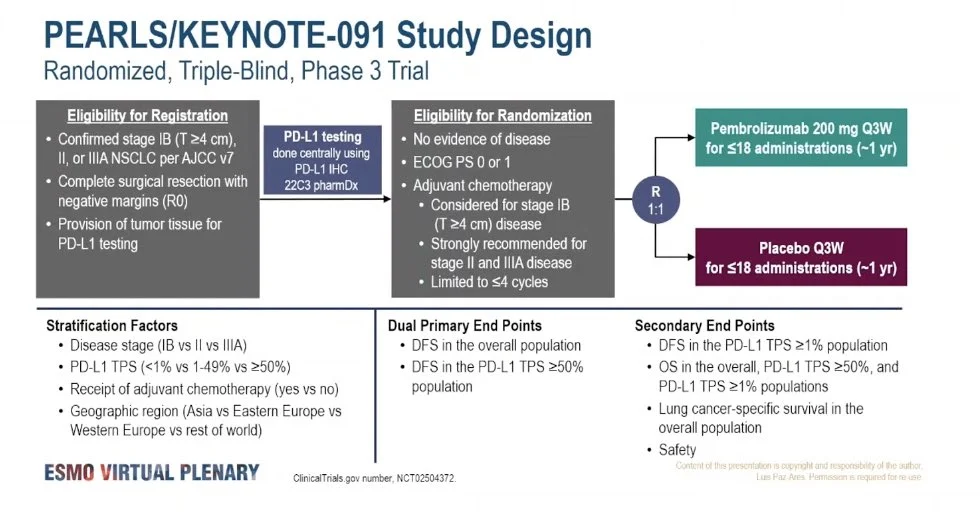
The pivotal study evaluating pembrolizumab in resected NSCLC is KEYNOTE-091 (PEARLS), a randomized, double-blind, phase III trial.
Study Design
- Population: Completely resected stage IB (≥4 cm), II, or IIIA NSCLC
- Prior adjuvant chemotherapy: Allowed and commonly administered
- Intervention: Pembrolizumab for up to 1 year
- Comparator: Placebo
- Primary endpoints: Disease-free survival in
- The overall population
PD-L1 TPS ≥50% subgroup
Primary Endpoint: Disease-Free Survival (DFS)
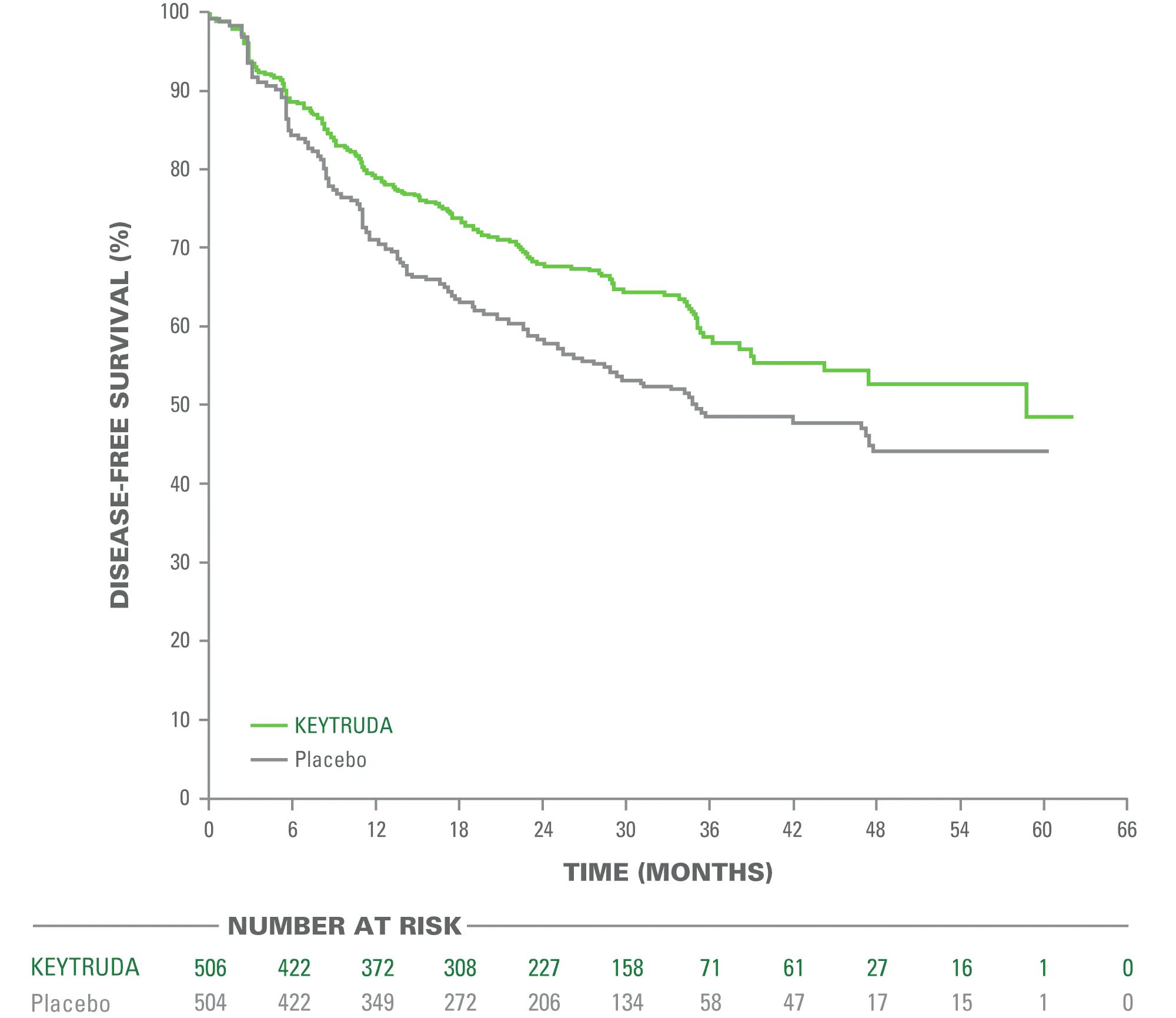
The trial demonstrated a statistically significant improvement in DFS in the overall study population:
- Median DFS:
53.6 months with pembrolizumab
vs 42.0 months with placebo - Hazard Ratio (HR): 0.76
- 95% Confidence Interval (CI): 0.63–0.91
- P-value: 0.0014
This established adjuvant pembrolizumab as an evidence-based option following resection.
PD-L1–Stratified Outcomes
KEYNOTE-091 was designed with two primary endpoints:
- Disease-free survival (DFS) in the overall study population, and
- DFS in the PD-L1 tumor proportion score (TPS) ≥50% subgroup.
While pembrolizumab significantly improved DFS in the overall population, the results within PD-L1–defined subgroups were more complex.
PD-L1 ≥50%
In patients with PD-L1 TPS ≥50%, pembrolizumab showed a numerical reduction in the risk of recurrence compared with placebo. However, at the time of the primary analysis, this difference did not meet the predefined threshold for statistical significance for the co-primary endpoint.
Importantly, this does not indicate absence of benefit. Rather, it means that the study did not conclusively demonstrate a statistically confirmed advantage in this specific subgroup at that time point.
PD-L1 1–49% and <1%
Across PD-L1 subgroups (≥50%, 1–49%, and <1%), the magnitude of benefit did not follow a clear enrichment pattern, unlike what is typically observed in metastatic NSCLC.
In particular, within the PD-L1 <1% subgroup:
- Hazard ratios did not demonstrate a clearly robust or statistically definitive benefit.
- Analyses were exploratory and not powered to confirm predictive value.
Accordingly: The benefit for patients with PD-L1 <1% is unclear.
How Do We Apply This in Clinical Practice?
Based on current evidence:
After complete resection (stage IB ≥4 cm – IIIA):
- Adjuvant platinum chemotherapy remains standard for eligible stage II–IIIA patients.
- Adjuvant pembrolizumab for up to 1 year is an evidence-supported option following resection (with or without prior chemotherapy).
- PD-L1 testing informs discussion but is not an absolute gatekeeper.
However, in patients with PD-L1 <1%, treatment decisions require careful consideration because:
- The magnitude of benefit is uncertain.
- PD-L1 was not a strong predictive biomarker in this trial.
- Risk–benefit balance must consider stage, nodal status, comorbidities, and patient preferences.
Conclusion
Conclusion
Adjuvant pembrolizumab, supported by phase III evidence from KEYNOTE-091, represents a meaningful addition to the management of completely resected NSCLC. The trial demonstrated a statistically significant improvement in disease-free survival in the overall study population, establishing pembrolizumab as a valid adjuvant therapeutic option following surgery (with or without prior platinum-based chemotherapy).
However, the predictive role of PD-L1 expression in this setting remains complex. Unlike in metastatic NSCLC, PD-L1 did not demonstrate a consistent enrichment pattern for benefit in the adjuvant context. In particular:
The benefit for patients with PD-L1 <1% is unclear.
This subgroup continues to represent the greatest area of clinical uncertainty, where treatment decisions must be individualized based on stage, risk of recurrence, comorbidities, and patient preference. These findings underscore the need for more refined biomarkers and longer-term survival data to optimize patient selection in early-stage NSCLC.
You Can Watch More on OncoDaily Youtube TV