Immune checkpoint inhibitors (ICIs) targeting the PD-1/PD-L1 axis have dramatically altered the treatment landscape of advanced non small cell lung cancer (NSCLC). While monotherapy and chemo-immunotherapy combinations are widely adopted, the role of dual checkpoint inhibition—specifically combining PD-1/PD-L1 with CTLA-4 blockade—remains an area of active investigation.
The question persists: does dual ICI offer added benefit over single-agent ICI, and in which patient subgroups?
This reconstructed individual patient data (IPD) meta-analysis by Di Federico et al. synthesizes survival outcomes across multiple phase III trials to assess whether specific biomarkers, such as PD-L1 tumor proportion score (TPS) and STK11 mutations, can identify patients who derive long-term benefit from dual checkpoint blockade.

Methods and Study Design
A systematic review was conducted across PubMed, MEDLINE, and Embase to identify randomized phase III trials investigating dual CTLA-4 plus PD-1/PD-L1 inhibition versus single-agent PD-1/PD-L1 inhibition in treatment-naive advanced NSCLC patients. Eligible trials included those with:
- Advanced/metastatic NSCLC without oncogenic driver mutations.
- Use of ICI ± chemotherapy versus chemotherapy.
- Kaplan–Meier data available for ≥5-year OS or subgroup analyses.
Six RCTs were included: CheckMate 227, CheckMate 9LA, POSEIDON, Keynote-189, Keynote-407, and IMpower150. Individual patient data were reconstructed from survival curves using WebPlotDigitizer and IPDfromKM. Subgroup analyses evaluated outcomes based on PD-L1 TPS (<1% vs ≥1%), tumor histology, and KRAS/STK11/KEAP1 mutational status.
Key Results
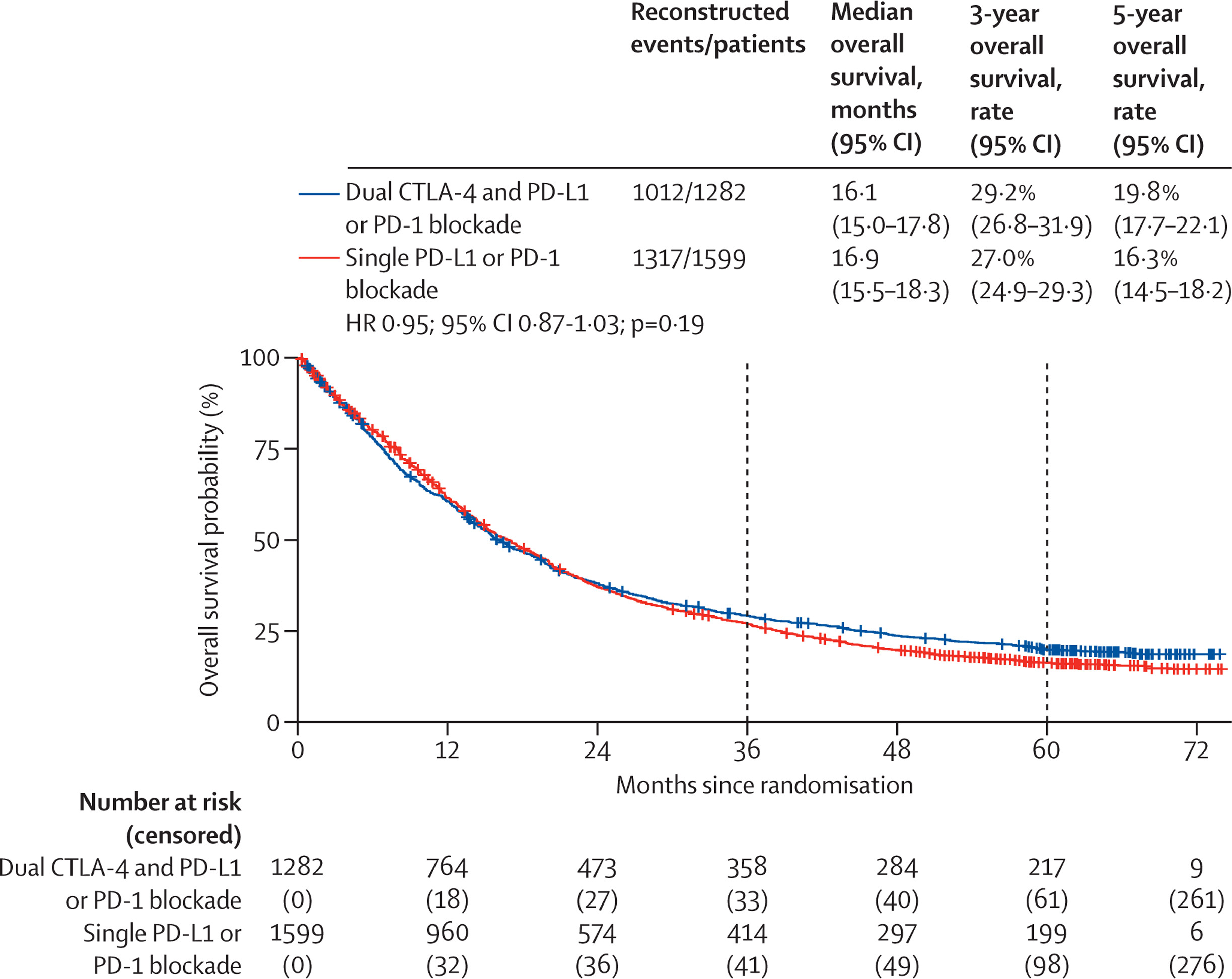
Overall Survival in the General Population
Median overall survival (OS) was comparable between treatment groups:
- Dual ICI: 16.1 months
- Single ICI: 16.9 months
(Hazard ratio [HR] = 0.95; p = 0.19)
Five‑year OS rates were 19.8% with dual immune checkpoint blockade and 16.3% with single-agent therapy. Although the difference in median OS was not statistically significant, time-dependent modeling revealed a late survival benefit for dual therapy. This suggests that the combination may deliver more durable immune responses in a subset of long-term survivors.
Subgroup Analyses: Who Benefits Most from Dual ICI?
In a deeper look at patient subgroups, the study examined key biomarkers to determine who derives the greatest benefit from dual immune checkpoint blockade.
PD-L1 Expression Subgroups
For patients with low PD-L1 expression (TPS <1%), dual immune checkpoint inhibition significantly improved overall survival. The median OS increased from 14.5 months (single-agent ICI) to 15.5 months (dual ICI), with a hazard ratio of 0.85 (p = 0.021). Importantly, 5-year survival nearly doubled from 9.3% to 16.6%, highlighting a notable long-term benefit in a population typically resistant to immunotherapy.
In contrast, among patients with PD-L1 TPS ≥1%, there was no significant OS difference between single-agent and dual ICI treatment (HR: 0.97, p = 0.60). These findings suggest that for PD-L1–high tumors, single-agent immunotherapy may be sufficient, avoiding additional toxicity associated with dual blockade.
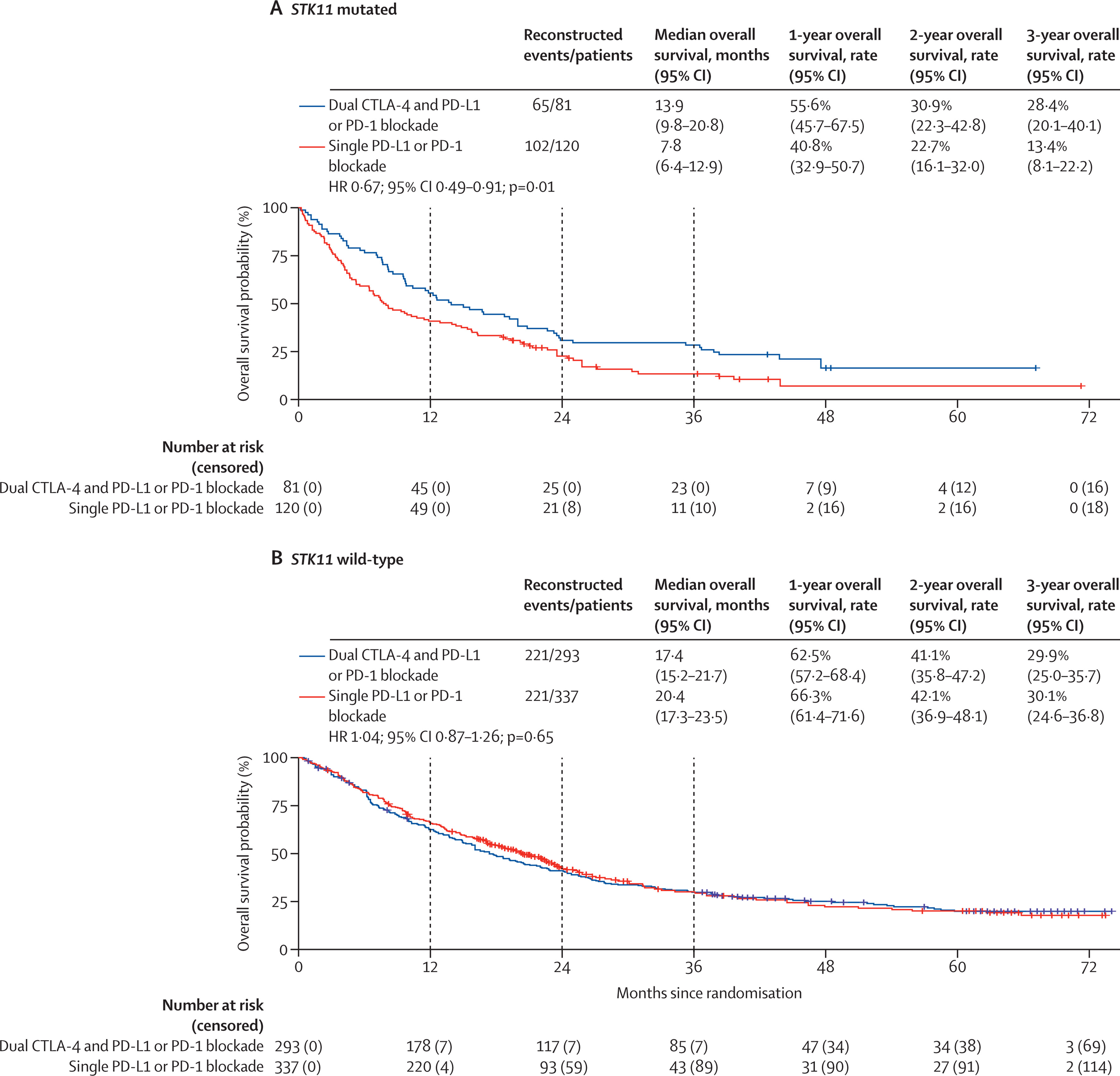
STK11-Mutated Tumors
Patients harboring STK11 mutations—known to create immunologically “cold” tumor microenvironments—demonstrated a clear benefit from dual ICI. Median OS improved from 7.8 to 13.9 months, with a hazard ratio of 0.67 (p = 0.012). The proposed mechanism is that CTLA-4 inhibition may help overcome immune exclusion by enhancing CD4⁺ T cell priming and macrophage-driven cytotoxicity, making this subgroup especially responsive to combination therapy.
KRAS and KEAP1 Mutations
In patients with KRAS mutations, no overall survival benefit was seen with dual versus single ICI, likely reflecting the biological heterogeneity of KRAS-driven cancers and the influence of co-mutations.
Similarly, patients with KEAP1 mutations did not benefit from dual immunotherapy. This resistance aligns with prior data suggesting that KEAP1 loss is associated with impaired antitumor immunity and resistance to checkpoint blockade.
Tumor Histology
No significant OS difference was observed between squamous and non-squamous histologies, challenging previous meta-analyses that reported greater ICI benefit in squamous NSCLC. This discrepancy may be due to differences in trial design or the confounding effects of chemotherapy combinations in earlier analyses.
Clinical Interpretation of Dual ICI Use in NSCLC
This meta-analysis sheds light on which NSCLC patient subgroups might truly benefit from dual immune checkpoint blockade (typically PD-1 + CTLA-4 inhibition) and offers practical guidance for treatment personalization.Patients with PD-L1 tumor proportion score (TPS) below 1% showed a clear overall survival (OS) benefit from dual ICI therapy. In this setting, where single-agent PD-1 inhibitors often underperform, intensifying treatment with dual ICI is clinically reasonable.
Similarly, STK11-mutated tumors, which are traditionally considered immunologically “cold” and resistant to single-agent ICI, demonstrated significant OS benefit with dual checkpoint inhibition. This subgroup may warrant prioritization for combination immunotherapy strategies.
On the other hand, KRAS-mutated and KEAP1-mutated tumors—despite their high prevalence—did not derive additional benefit from dual ICI compared to monotherapy. For KRAS-mutant NSCLC, a standard PD-1 inhibitor ± chemotherapy remains the preferred approach. KEAP1-mutated tumors may require alternative strategies, and further prospective studies are needed to guide immunotherapy intensification in this group.
Interestingly, for patients with PD-L1 expression ≥1%, dual ICI conferred no added survival benefit over monotherapy. In these cases, especially with high PD-L1 expression, single-agent PD-1/PD-L1 inhibitors remain the optimal, less toxic choice.
Mechanistic Rationale
A 2024 preclinical study (ref. 13) provided a compelling mechanistic rationale for targeting CTLA-4 in STK11/KEAP1-mutated non–small cell lung cancer (NSCLC)—a molecularly defined subgroup typically resistant to PD-1/PD-L1 blockade. In this model, CTLA-4 inhibition reprogrammed the immunosuppressive tumor microenvironment by activating CD4⁺ T cells, recruiting iNOS⁺ macrophages, and enhancing MHC class II expression on tumor-associated macrophages (TAMs). These coordinated immune shifts transformed otherwise immunologically “cold” tumors into inflamed, treatment-responsive ones—suggesting that CTLA-4 blockade may overcome primary resistance mechanisms in this context.
Despite these promising insights, the analysis faced notable limitations. The study relied on indirect reconstruction of individual patient data (IPD) rather than access to raw-level datasets, introducing potential bias. Furthermore, the mutational subgroup analyses, particularly those involving KEAP1 alterations, were underpowered due to the rarity of these events. The inclusion of data from heterogeneous sequencing platforms (e.g., blood-based vs tissue-based genotyping) further complicated interpretation. Additionally, the study lacked the ability to stratify outcomes by KRAS isoform—a relevant omission, given the differential biology and therapeutic implications of variants such as G12Cversus G12D.