The IMvigor011 trial, presented by Prof. Thomas B. Powles at the ESMO Congress 2025 in Berlin, demonstrated that ctDNA-guided adjuvant treatment with atezolizumab significantly improved both disease-free survival (DFS) and overall survival (OS) compared with placebo in patients with high-risk muscle-invasive bladder cancer (MIBC) following radical cystectomy. This landmark phase 3 study establishes ctDNA-based molecular residual disease (MRD) monitoring as a clinically validated approach to identify patients who benefit from adjuvant immunotherapy, marking a major step toward personalized treatment in bladder cancer.
Background
The IMvigor011 trial represents the first global, randomized, double-blind phase 3 study designed to evaluate a ctDNA-guided adjuvant immunotherapy strategy in patients with high-risk muscle-invasive bladder cancer (MIBC) following radical cystectomy. Despite advances in perioperative therapy, a significant proportion of patients with MIBC experience disease recurrence after surgery due to undetected molecular residual disease (MRD). Conventional imaging often fails to identify minimal residual disease early enough to intervene effectively.
By contrast, circulating tumor DNA (ctDNA) analysis enables the noninvasive detection of microscopic disease burden in the bloodstream, offering a window into tumor persistence long before radiographic relapse. IMvigor011 was therefore conceived to test whether real-time ctDNA monitoring could not only detect patients at highest risk of recurrence but also guide adjuvant immunotherapy with atezolizumab in a biomarker-driven, precision oncology framework.
Methods
A total of 761 patients with histologically confirmed, high-risk MIBC and no radiographic evidence of disease following radical cystectomy were enrolled in a structured ctDNA-based surveillance program. Enrollment occurred between 6 and 24 weeks post-surgery, during which patients underwent serial ctDNA testing for up to one year to assess for MRD.
Patients who tested ctDNA-positive (ctDNA⁺)—indicating the presence of residual microscopic disease—were randomized in a 2:1 ratio to receive either atezolizumab 1680 mg or placebo, administered every 4 weeks for 12 cycles (up to one year). The primary endpoint of the study was investigator-assessed disease-free survival (DFS), with overall survival (OS) as a key secondary endpoint under alpha control.
Patients who persistently tested ctDNA-negative (ctDNA⁻) throughout the surveillance period were not given adjuvant treatment, allowing investigators to evaluate the natural course of disease in individuals without detectable molecular recurrence. This design provided a unique opportunity to determine whether ctDNA negativity could reliably identify patients who might safely avoid additional therapy while maintaining excellent outcomes.
Results
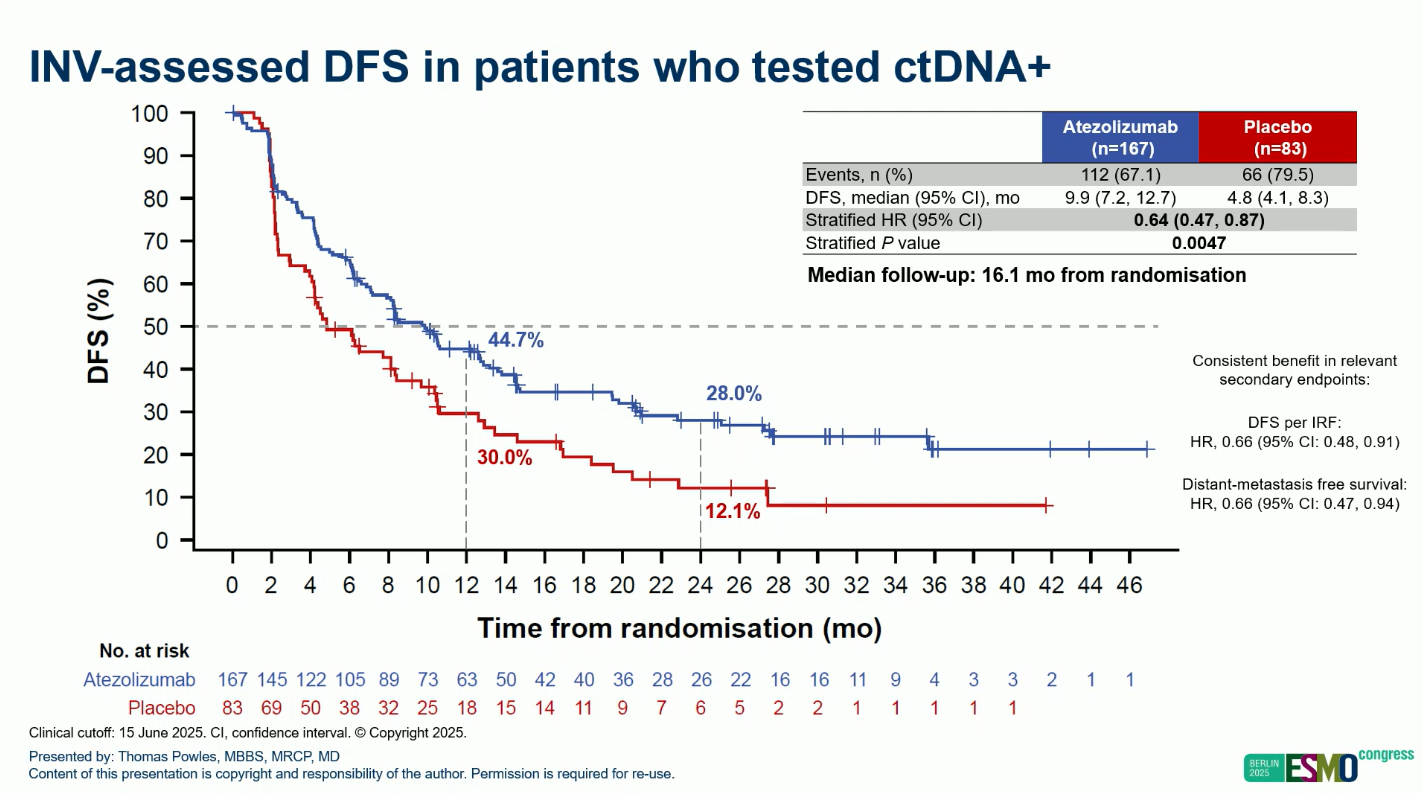
Out of 761 patients, 250 were ctDNA+ and randomized (atezolizumab, n=167; placebo, n=83).
At a median follow-up of 16.1 months, results demonstrated:
- DFS: HR 0.64 (95% CI 0.47–0.87; P=0.0047)
- Median DFS: 9.9 months (atezo) vs 4.8 months (placebo)
- 12-month DFS rate: 44.7% vs 30.0%
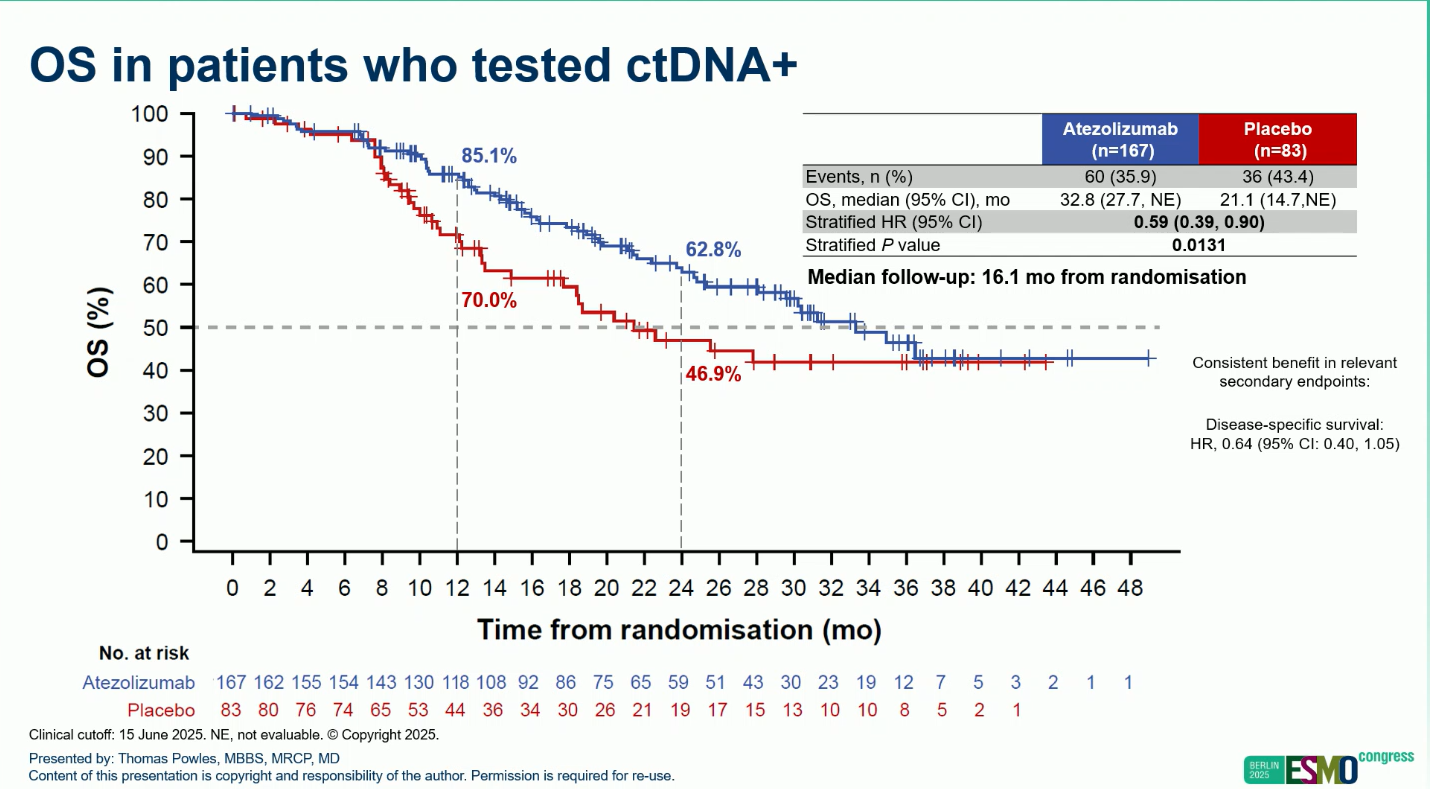
- OS: HR 0.59 (95% CI 0.39–0.90; P=0.0131)
- Median OS: 32.8 months vs 21.1 months
- 12-month OS rate: 85.1% vs 70.0%
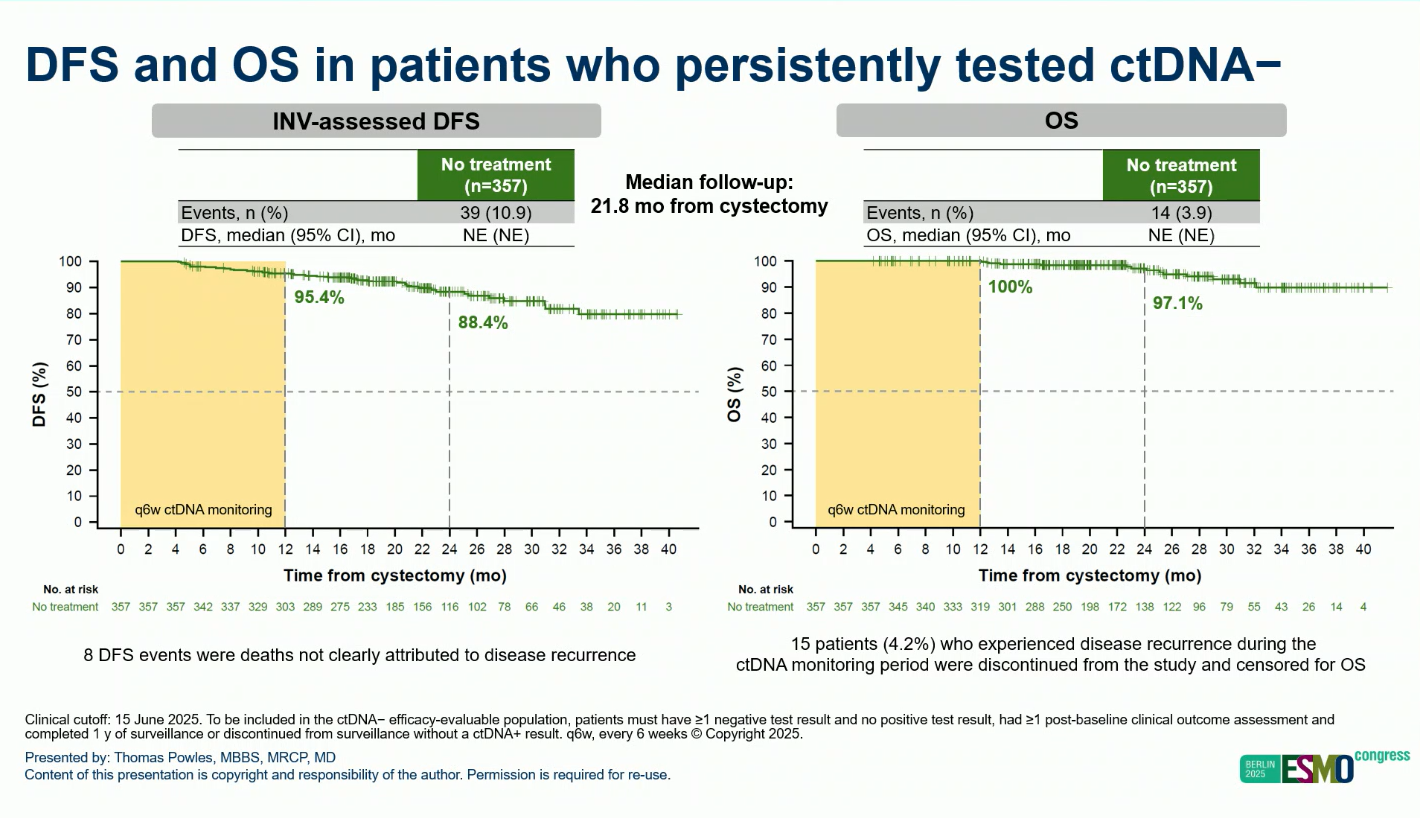
Among 357 ctDNA negative patients, the DFS rate was 95.4% at 1 year and 88.4% at 2 years, indicating low recurrence risk without adjuvant therapy.
Adverse events (AEs) were consistent with the known safety profile of atezolizumab
Conclusions
The IMvigor011 trial, presented by Prof. Thomas B. Powles at the ESMO Congress 2025 in Berlin, showed that ctDNA-guided adjuvant atezolizumab significantly improved both disease-free survival (DFS) and overall survival (OS) versus placebo in patients with ctDNA-positive, high-risk muscle-invasive bladder cancer (MIBC) following cystectomy. The findings validate ctDNA as a powerful biomarker for guiding post-surgical immunotherapy decisions, offering a practical framework for precision oncology in bladder cancer.
Notably, patients who remained ctDNA-negative had a good prognosis without additional treatment, underscoring the potential of ctDNA surveillance to not only identify those who benefit from adjuvant therapy but also to spare others from unnecessary toxicity. This pivotal study establishes IMvigor011 as a key milestone in the movement toward personalized, biomarker-driven adjuvant strategies in uro-oncology.
You can read the full abstract here.


