At OncoDaily Immuno-Oncology, this week’s expert voices capture the evolving reality of cancer immunotherapy — from real-world evidence challenging standard immune checkpoint inhibitor dosing in gastric cancer and new regulatory milestones for durvalumab in endometrial cancer, to pivotal trial readouts in melanoma, emerging strategies for ICI rechallenge in urothelial carcinoma, and combination IO platforms redefining genitourinary cancer care.
Alongside these clinical and translational advances, thought leaders reflect on the future of engineered T-cell therapies, biomarker-driven lung cancer biology, oncolytic peptide innovation, ultra-low-dose immunotherapy paradigms, and fundamental mechanisms of immune suppression in lung tumors — together shaping the scientific, clinical, and strategic direction of the next era in immuno-oncology.
This Week’s Expert Highlights in Immuno-Oncology
Oscar Tahuahua (Medical Oncology Fellow at the National Cancer Institute of Mexico):
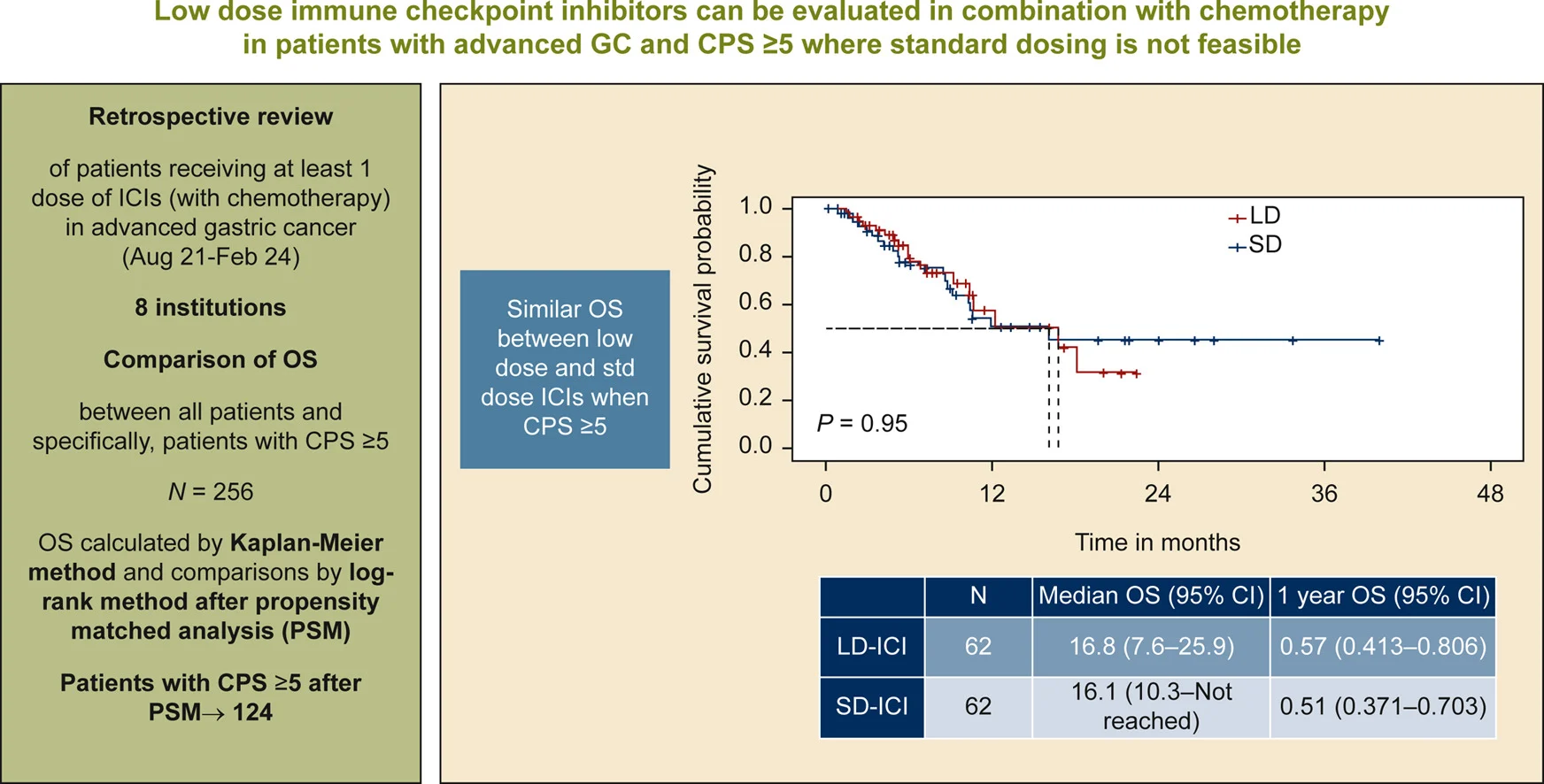
” In advanced MSS gastric cancer (CPS ≥5), low dose ICIs + chemo showed similar OS (16.8 vs 16.1 mo) to standard dosing in RW data , with biological rationale supported by early PD1 receptor saturation.
Low dose immunotherapy continues to challenge standard dosing paradigms, with prospective evaluation pending.”
Ayushi Pareek (Assistant Manager Business Development at Akums Drugs & Pharmaceuticals Ltd):
“AstraZeneca Pharma India receives CDSCO approval for an additional indication of Imfinzi (Durvalumab solution for infusion) for expanding treatment options for patients with advanced or recurrent endometrial cancer in India.
This milestone reinforces the growing role of immuno-oncology in improving cancer outcomes and highlights continued progress in bringing innovative therapies to patients who need them most.
A meaningful step forward for oncology care in India.”

Adnan Khattak (Consultant Medical Oncologist at Fiona Stanley Hospital and Hollywood Private Hospital):
“KeyVibe-010, Phase III trial, evaluating vibostolimab co-formulated with pembrolizumab as adjuvant therapy in resected stage IIB–IV melanoma has unfortunately not demonstrated additional clinical benefit compared to pembrolizumab alone.
Another difficult outcome, following earlier premature discontinuations or negative studies such as PIVOT-12 and RELATIVITY-098. While these results are disappointing, they reinforce an essential truth in oncology: clinical trials are the only way we truly discover what works — and what does not.
Negative trials are not failures. They are important data. They refine our understanding, redirect scientific effort, protect future patients from ineffective strategies, and shape the next generation of research questions. Timely and transparent reporting of such results is critical for scientific progress.
We are deeply grateful to the patients who participated in this study — your contribution advances knowledge for the global oncology community.
At One Clinical Research, we are proud to have been the lead recruiting site globally for this trial. The commitment of our patients, investigators Azim Khan,Omar Faruque and research team remains unwavering. We value our collaboration with MSD, MSD Australia and New Zealand Moderna.
Now, we turn our focus with cautious optimism toward INTERPATH-001, the personalised cancer vaccine trial. After consecutive setbacks in the adjuvant melanoma space, we hope this next chapter brings meaningful progress for our patients.
The work continues. Always.”
Diego A. Díaz García (Medical Oncologist, CEO and Founder at CánCare – Advanced Specialty in Oncology):
“ICI Rechallenge in Advanced Urothelial Carcinoma.
In a cohort of 35,789 patients, 292 (0.81%) received ≥2 ICI-containing lines between 2014 and 2024. Bladder cancer accounted for 86%, upper tract 14%. At diagnosis, 49% had stage IV disease.
Median prior ICI duration: 19.5 months.
Median interval to rechallenge: 9.4 months.
Median follow-up: 21.7 months.
Outcomes with ICI rechallenge:
- Median OS: 20.5 months
- Median PFS: 10.3 months
Key associations:
- Prior ICI in the nonmetastatic setting correlated with longer OS compared with both lines delivered in advanced disease, P < 0.001.
- In metastatic pretreated patients, an interval ≥12 months before rechallenge was associated with improved OS, P = 0.027.
- Anti PD-1 following prior anti PD-L1 achieved the longest rechallenge duration, 18.2 months, P < 0.001.
Limitations include retrospective design, small rechallenge cohort, and potential residual confounding despite propensity score matching.
ICI rechallenge may offer meaningful benefit in selected patients, particularly those with earlier-stage exposure or longer ICI-free intervals. Prospective validation is warranted before broader adoption. ”
Jack Shuang Hou (Executive, Diagnostic Technology Firm – Regulatory & Business Development):
“Merck Presents KEYTRUDA +Padcev and WELIREG Combinations at ASCO GU 2026 — Expanding Standards of Care in Bladder and Kidney Cancer
At the 2026 American Society of Clinical Oncology (ASCO) GU Cancers Symposium, Merck and Co., Inc. unveiled multiple late-breaking datasets across genitourinary cancers — and the message is clear: combination immuno-oncology is moving earlier and becoming the backbone of therapy.
Dr. Marjorie Green — Senior Vice President & Head of Oncology, Global Clinical Development, Merck Research Laboratories — highlighted new results spanning both approved and investigational programs.
Key clinical signals:
Bladder cancer — perioperative immunotherapy becomes real
KEYNOTE-B15 showed:
- KEYTRUDA (pembrolizumab) + Padcev (enfortumab vedotin-ejfv) → improved event-free survival, overall survival, and pCR rates in muscle-invasive disease
This pushes IO + ADC therapy from metastatic → curative-intent setting.
Kidney cancer — triplet and targeted IO era
- WELIREG (belzutifan) + KEYTRUDA improved DFS (LITESPARK-022)
- WELIREG + LENVIMA (lenvatinib) improved PFS (LITESPARK-011, collaboration with Eisai Co., Ltd.)
The HIF-2α inhibitor class is clearly moving toward backbone combination status.
Next-gen pipeline emerging
- sacituzumab tirumotecan ADC with Kelun-Biotech
- multiple KEYMAKER and LITESPARK expansion studies
My takeaway
The oncology playbook is changing again:
- Checkpoint inhibitor monotherapy → combination IO
- Combination IO → biologically targeted IO backbone
- Metastatic success → perioperative & curative intent
We’re entering an era where drug classes matter less than mechanism synergy — ADC + IO + targeted metabolism inhibition are being engineered together as treatment systems, not standalone therapies.
Companies winning the next decade in oncology won’t just have a drug.
They’ll have a programmable combination platform.”

Emily VonAldenbruck (Biotech Communications | Immunotherapy Advocate | Cancer Awareness Content Creator):
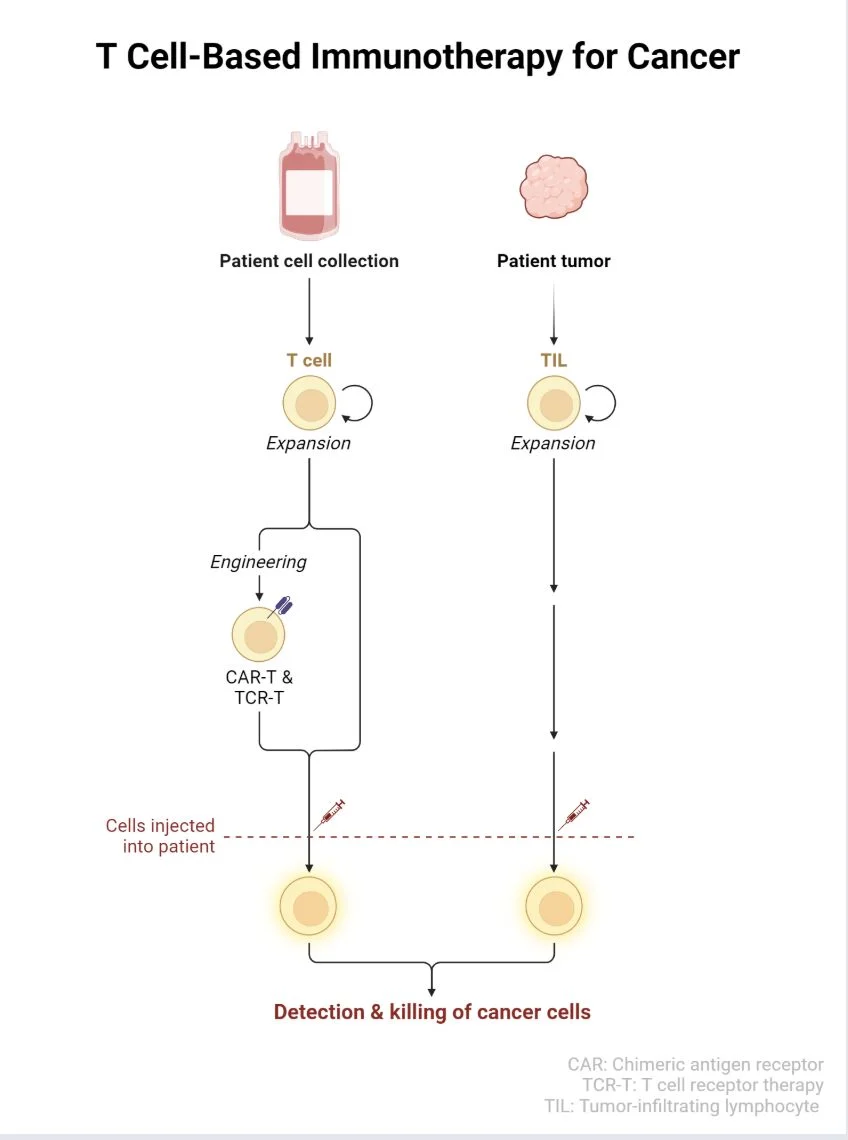
“T cell immunotherapy still blows my mind a little
Instead of just giving drugs and hoping for the best, we take a patient’s own T cells, grow them, tweak them (CAR-T, TCR-T, or TILs), and send them back into the body to do what they were originally meant to do: recognize and kill cancer.
The process is surprisingly elegant:
- collect T cells from blood or tumor
- expand and engineer them in the lab
- infuse them back into the patient
- let the immune system take over
What’s crazy is how targeted this can be.
These cells aren’t just attacking everything — they’re trained to recognize specific tumor signals.
Of course, it’s not perfect.
Solid tumors are harder to treat, tumors can change their antigens, and side effects are real.
But still…
The idea that we can “teach” the immune system how to fight cancer feels like a huge shift in how we think about treatment
It makes cancer feel less like an unbeatable enemy and more like a problem we’re slowly learning how to outsmart.”
Akhil Santhosh (Medical Oncology Specialist at MVR Cancer Centre and Research Institute):
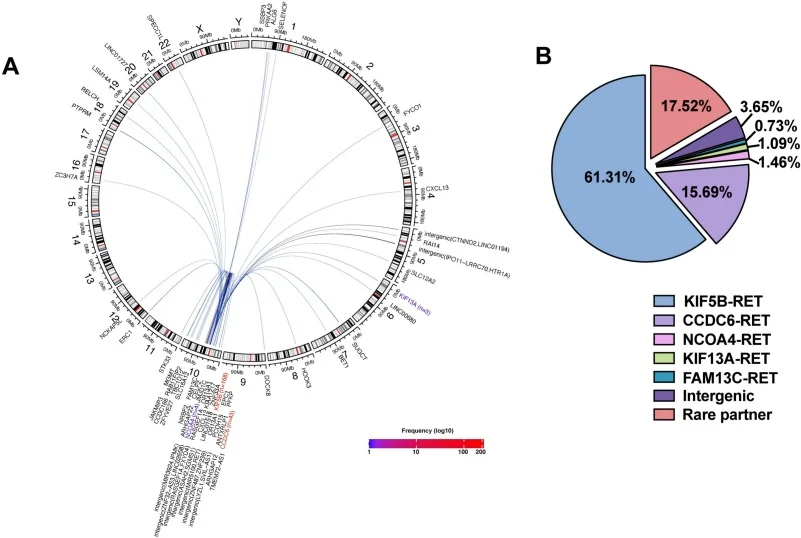
” RET Fusion–Positive lung Adenocarcinoma: Partner-Specific clinicopathological Characteristics, co-mutation Profiles, and Implications for targeted and Immunotherapy – Lung Cancer”
Lorenzo Galluzzi (Associate Professor at Fox Chase Cancer Center):
“Here comes our latest Journal for ImmunoTherapy of Cancer review on oncolytic peptides for cancer immunotherapy. Kudos to Oliver for doing the hard lifting here. ”
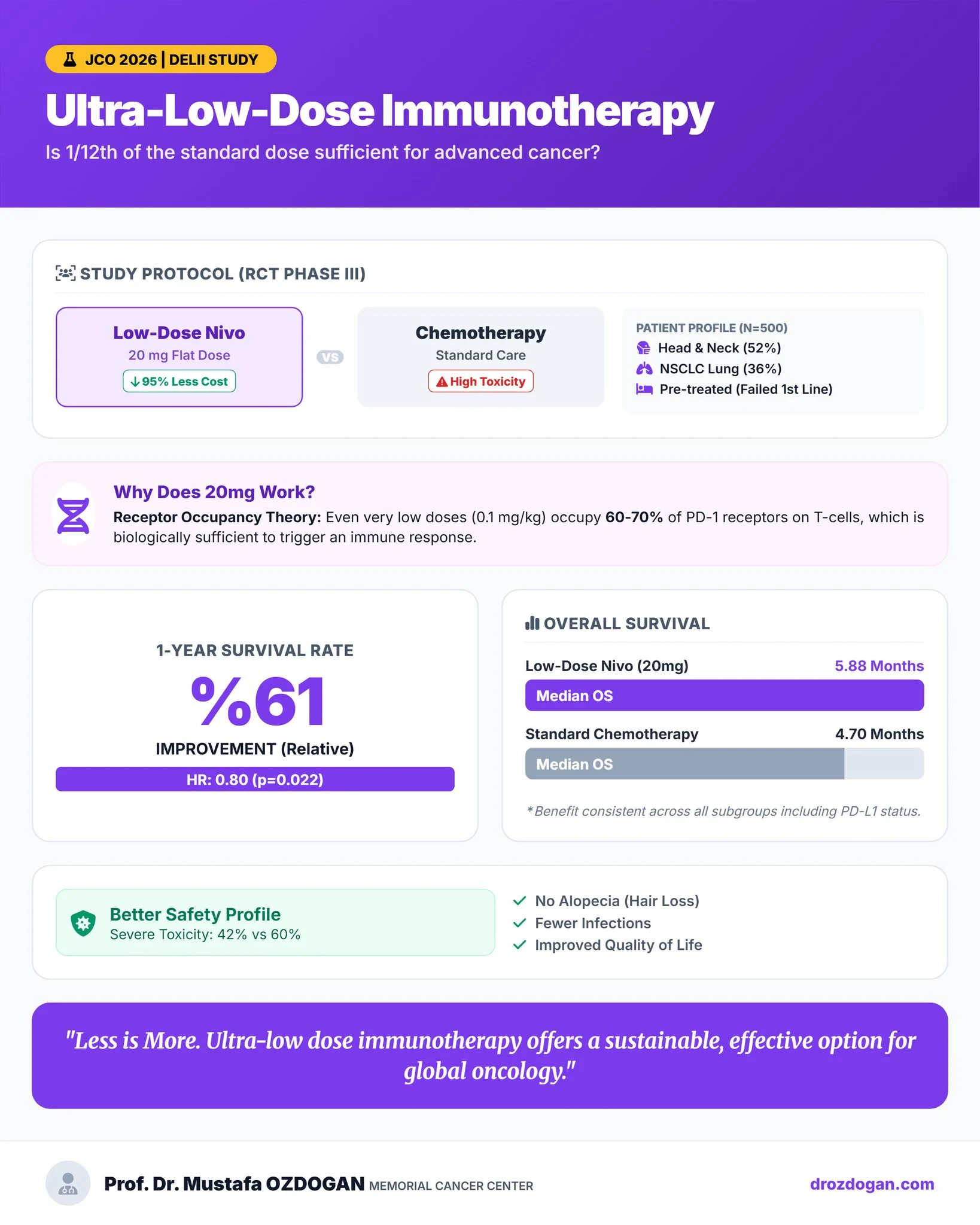
Mustafa Özdoğan (Head of Division of Medical Oncology at Memorial Cancer Center):
“Breaking in Global Oncology: The DELII Study Can 1/12th of the standard immunotherapy dose be just as effective? The DELII Study (Phase III RCT) recently published in JCO provides a compelling “Yes” for advanced cancer care.
Read the full study ”
Igor Santiago-Carvalho (Ph.D in Immunology, Senior Research Fellow at Mayo Clinic):
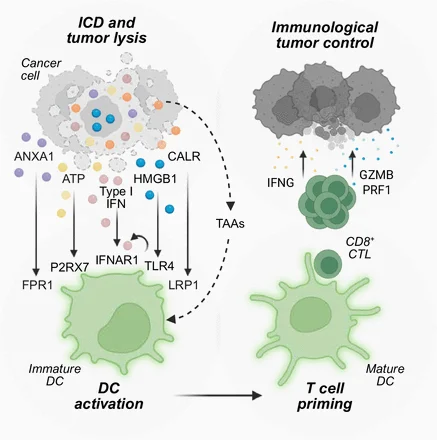
“Our recent research paper “Regulatory T-cell sensing of extracellular ATP via P2RX7 promotes their accumulation and suppression and drives lung tumor growth” was included in the Accelerating Cancer Immunotherapy Research newsletter this week”



