This week in OncoDaily Immuno-Oncology, expert voices across translational science, clinical practice, and emerging technology are deepening our understanding of how genetic context, tumor microenvironment modeling, and next-generation cell engineering shape immunotherapy response. From CRISPR-defined CHD1/MAP3K7 vulnerabilities predicting checkpoint sensitivity and immunocompetent ovarian cancer platforms enabling precision combination strategies, to CER-T cells introducing engulfment-based antitumor immunity and ctDNA refining real-time treatment monitoring, the field continues to expand beyond conventional paradigms. Real-world toxicity management, cost-effectiveness analyses, immunoinformatics innovation, and personalized vaccine concepts further highlight a rapidly converging landscape where biology, technology, and clinical insight collectively drive more precise, adaptive, and globally impactful cancer immunotherapy.
This Week’s Expert Highlights in Immuno-Oncology
Yan Leyfman (Medical Oncologist, Co-Founder and Executive Director of MedNews Week):
” Why do only some patients respond to cancer immunotherapy?
A new study uncovers genetic vulnerabilities that may help explain—and predict—who benefits.
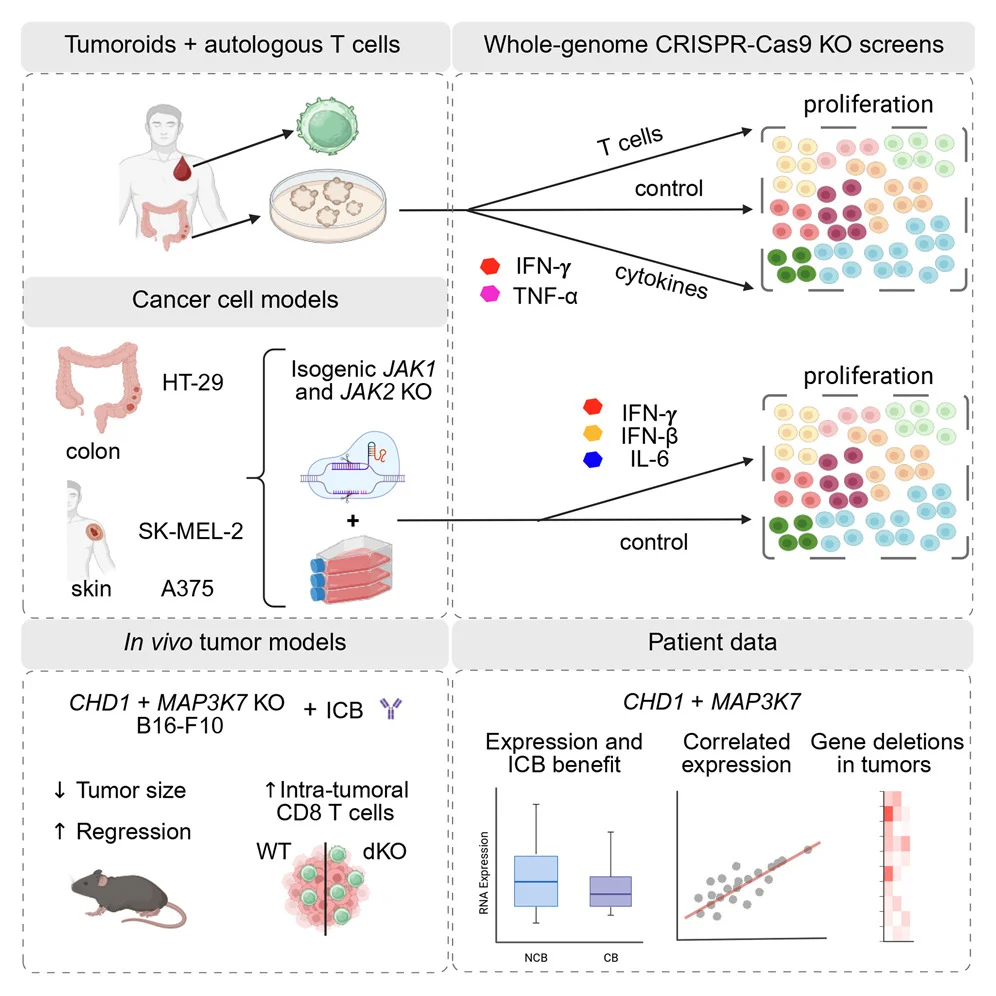
What was done: Using whole-genome CRISPR-Cas9 screens, researchers interrogated cancer–immune interactions across:
- Autologous tumoroid–T cell co-cultures
- Isogenic cancer models with impaired IFN signaling
- Multiple cytokine contexts
Key discovery: Loss of CHD1 or MAP3K7 (TAK1) amplifies IFN-γ–driven transcription, creating an acquired sensitivity to tumor-reactive T cells
In vivo relevance:
- Checkpoint blockade worked better in melanoma models lacking Chd1 or Map3k7
- Tumors showed more CD8⁺ T cells and higher activation
Clinical signal:
- CHD1 and MAP3K7 are recurrently mutated in human cancers
- Lower tumor expression correlates with improved response to immune checkpoint inhibitors
Why this matters: These genes emerge as candidate biomarkers of immunotherapy response and potential levers for combination strategies
Bottom line: Genetic context matters—and CHD1/MAP3K7 loss may prime tumors for immune attack”
Maxwell Sauder (Onco-dermatologist at Princess Margaret Cancer Centre):
“A great oncology-focused double header this Tuesday.
At lunch, I had the opportunity to present noon rounds at Humber River Health Cancer Care Clinic, discussing first-line treatment of advanced urothelial carcinoma with enfortumab vedotin plus pembrolizumab, with can a focus on recognizing and managing associated cutaneous toxicities. Thank you to Katherine Simoes at Pfizer and Anu Vig at Merck supporting, and especially to Raminder Grewal for moderating and making the session possible.
In the evening, I had the pleasure of presenting in person to a group of 30+ and virtually to the oncology team at Trillium Health Partners, Carlo Fidani Peel Regional Cancer Centre. We reviewed cutaneous toxicities associated with dostarlimab in endometrial cancer, along with broader approaches to immunotherapy-related skin adverse events. Thank you to Joanna Kowalski and Jessica Wilson at GSK for supporting this session.
Always rewarding to engage with multidisciplinary oncology teams on practical, real-world management of treatment-related toxicities, where dermatology and oncology truly intersect.”

Anniina Färkkilä (Tenure track Professor of Translational Gynecologic Oncology at University of Helsinki):
” New publication alert!
Immunotherapy has shown limited success in hashtag#OvarianCancer — yet some patients respond remarkably well. How can we better identify who will benefit and match the right immunotherapy to each patient?
We’re excited to share our latest study introducing a next-generation patient-derived model for high-grade serous ovarian cancer (HGSC) that brings us closer to truly personalized immunotherapies.
What did we do?
We developed immunocompetent patient-derived cultures (iPDCs) grown on a human omentum-based matrix that closely mimics the real tumor environment. This high-throughput platform integrates drug testing, histology, genomics, single-cell and spatial analyses — all in one system.
Why does this matter?
We show that iPDCs faithfully reproduced tumor genetics, tissue architecture, and — critically — retained the patient’s own immune cells. Treatment responses in the model strongly matched how patients responded in the clinic.
Key findings:
- Identified patient-specific therapeutic vulnerabilities in recurrent HGSC
- Discovered a promising new combination therapy: ATR inhibition combined with immunotherapy targeting Autotaxin
- Showed that ATR inhibition boosts CD8⁺ T cell activation and infiltration, particularly PD1⁺ T cells, through spatial interactions with stressed tumor cells
Big picture: Our iPDC platform offers a powerful ex vivo tool to accelerate precision oncology, bridge preclinical testing with patient outcomes, and guide rational combination therapies for clinical translation in ovarian cancer.
Read the full publication ”
Michel Frank Ferrazo (Clinical Research Analyst – Technical Training-3 at Hemostasis Laboratory – Unicamp):
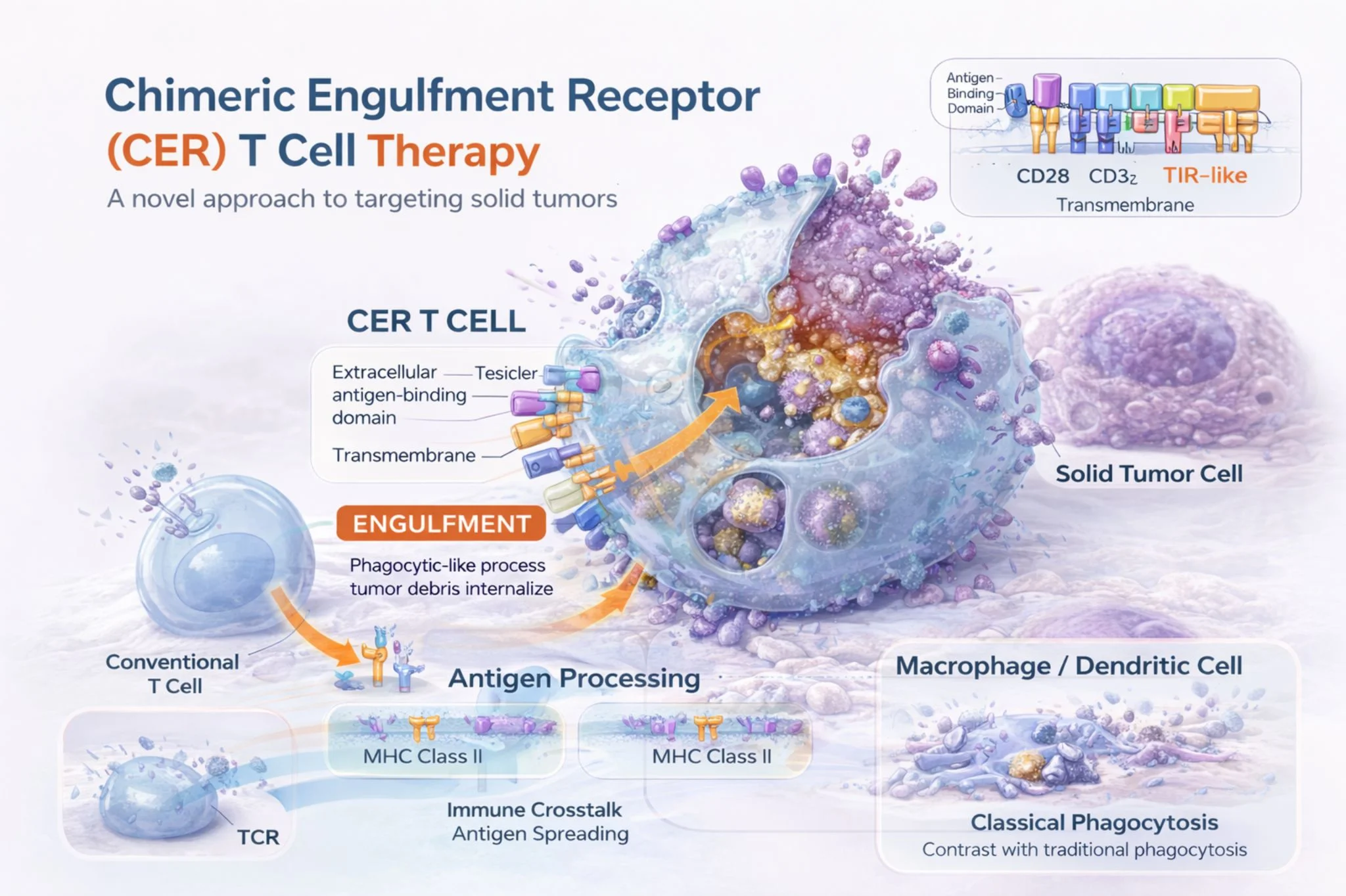
” Reimagining Cell Therapy and a friend with CAR-T: what if T cells could kill AND engulf tumor cells?
A 2023 Molecular Therapy study describes Chimeric Engulfment Receptor T cells (CER-T) engineered with a TIM-4 extracellular domain to recognize phosphatidylserine (PS) an “eat-me” signal frequently exposed on stressed tumor cells.
What’s compelling is the dual-function design:
- Cytotoxicity via CD28/CD3ζ signaling
- Phagocytic uptake (engulfment-like behavior) validated with inhibitor-sensitive assays
- APC-like cross-presentation, enabling activation of third-party antigen-specific T cells (HLA class I–dependent)
Even more interesting: standard targeted therapies may “prime” tumors by increasing PS exposure enhancing CER-T activity in models of:
- Mantle cell lymphoma (BTK inhibitor priming)
- EGFR-mutant NSCLC (osimertinib priming)
This is a shift from targeting a single classical antigen toward leveraging tumor stress biology with a potential “vaccine-like” amplification through cross-priming.
If this translates clinically, CER-T could represent a new class of engineered T cells that remove tumor cells directly and help broaden immunity indirectly.
Pashtoon Kasi (Medical Director of GI Medical Oncology at City of Hope Orange County):
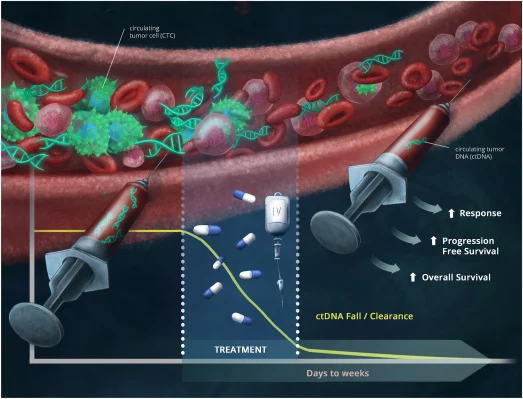
” Published today
“Utility of Circulating Tumor DNA-Based Liquid Biopsies in Patients with Cancer Receiving Immunotherapy.” From our fellow Maria Teixeira
ctDNA and focus on immunotherapy!
for 50 days. “
Roychoudhuri Lab (Professor of Cancer Immunology and Immunotherapy at the University of Cambridge):
“Immunoinformatics is revolutionising our understanding of cancer immunology and immunotherapy responses. If anyone is interested in joining our lab through this fellowship, I’d be very keen to support an application – do get in touch!”
Jason R. Williams (Chief of Interventional Oncology and Immunotherapy at Williams Cancer Institute):
“Cancer research is moving faster than most people realize. Russia just announced Enteromix, a personalized mRNA cancer vaccine showing promising early results in colorectal cancer patients. Tumor reduction of 60-80% in Phase I trials. The world is paying attention and they should be.
The concept of turning a patient’s own tumor into a personalized vaccine has been explored for quite some time. The approach involves ablating the tumor, then injecting combinations of immunotherapy agents directly into it. Checkpoint inhibitors, TLR agonists, vaccine adjuvants. The dead tumor cells become antigens and the immune system learns what to hunt. Not one target. Dozens. All unique to that patient’s cancer. Off-the-shelf vaccines typically target a single antigen. But cancer is smart. It mutates. It hides.
A single target is like having one photo of a suspect. When you use the patient’s own tumor as the vaccine, you give the immune system the photo, the fingerprints, the DNA, the scars, the tattoos. Good luck hiding from that. The entire field is moving toward a truth the science has pointed to for a long time: the cure for cancer is already within you. Your immune system just needs to be taught what to fight and where to fight it.
We are living in the most exciting era of cancer treatment in human history. This is the immunotherapy revolution and it’s accelerating. If you or someone you love is fighting cancer, know this. There are more options today than at any point in history.”
Pieter Garicano (Writing Silicon Continent, Editor at Works in Progress):
“Remarkable gap in survival rates in this new study where cancer patients were randomized to receive immunotherapy either before or after 3 p.m. Might be a big deal?”
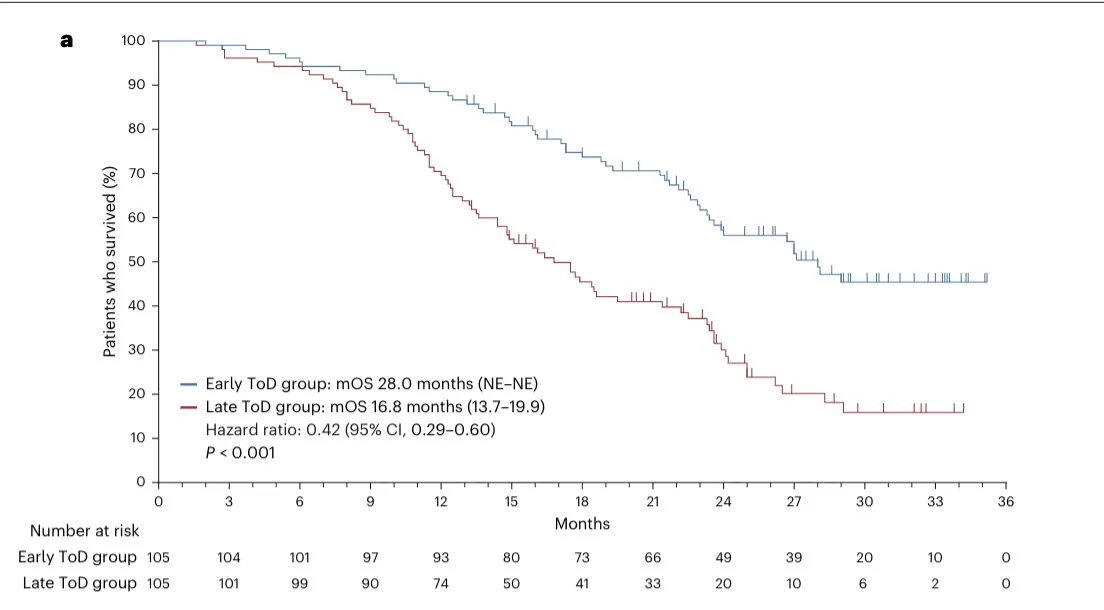
Erman Akkus (Medical Oncology Fellow at Ankara University):
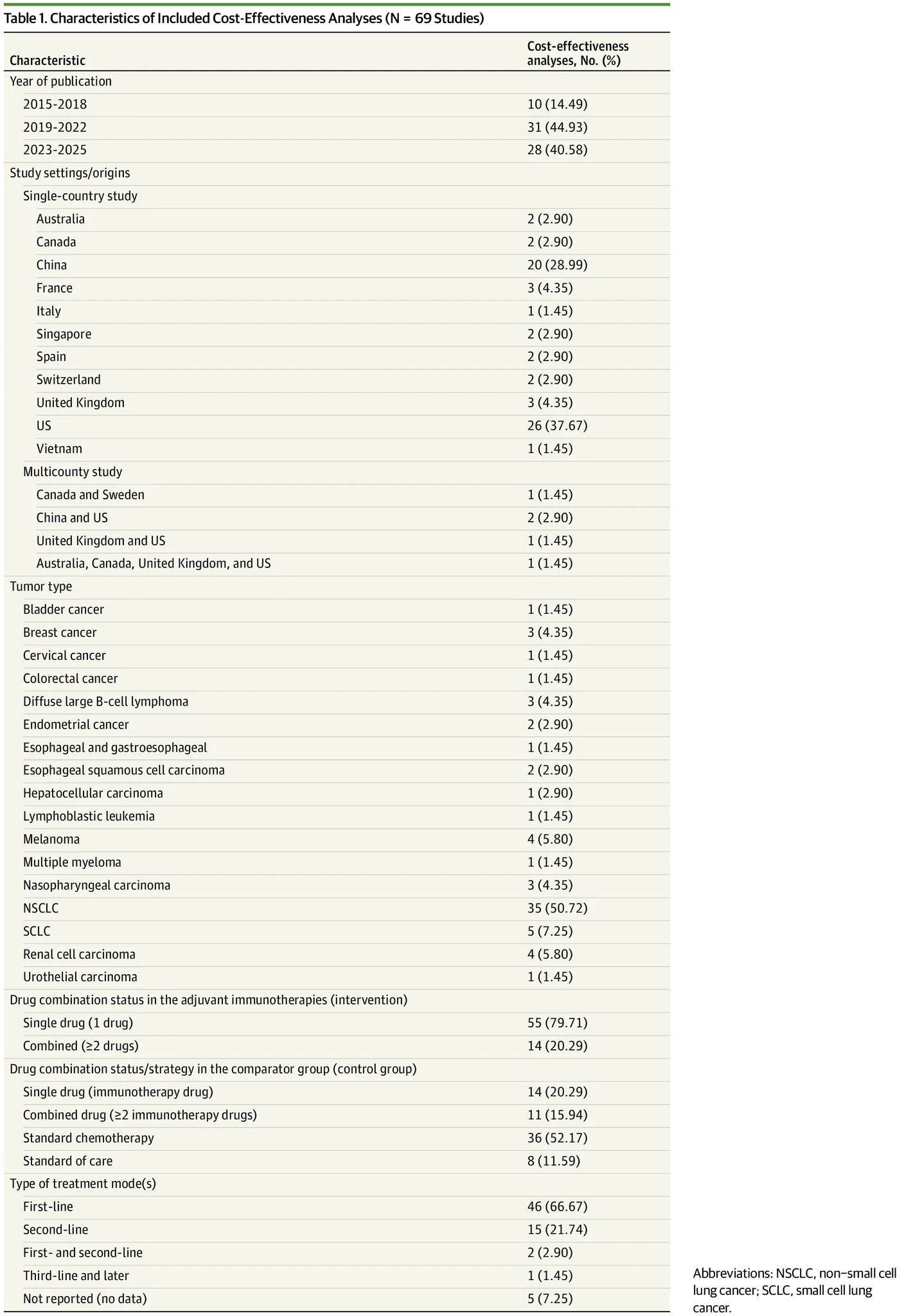
“Cost-Effectiveness of Adjuvant Immunotherapy in Cancer Treatments: A Systematic Review | Health Care Economics, Insurance, Payment | JAMA Oncology | JAMA Network ”
Jerome Cabeau (Business Development Manager at Thermo Fisher Scientific):
“In a groundbreaking advancement in cancer immunotherapy, researchers have unveiled the next-generation design of CAR-T cells that strategically leverage unique tumor features to enhance therapeutic efficacy. This innovative approach promises to significantly improve patient outcomes in the ongoing battle against”.


