Diffuse pleural mesothelioma (DPM) is a rare, aggressive malignancy of the pleura with ~30,000 new cases annually worldwide, strongly linked to asbestos exposure. Historically, outcomes have been poor, with little systemic therapy innovation until immune checkpoint blockade (ICB) demonstrated survival benefit in unresectable disease (CheckMate 743: nivolumab + ipilimumab; IND227: pembrolizumab + chemotherapy).
While perioperative strategies (surgery + chemotherapy ± radiotherapy) have been tested, randomized trials (MARS, MARS2) showed no survival benefit and high morbidity. Imaging-based response assessment is difficult due to mesothelioma’s growth pattern. Circulating tumor DNA (ctDNA) has emerged as a promising biomarker for residual disease detection and therapy monitoring, though it is technically challenging in mesothelioma due to low tumor mutation burden.

Methods and Study Design
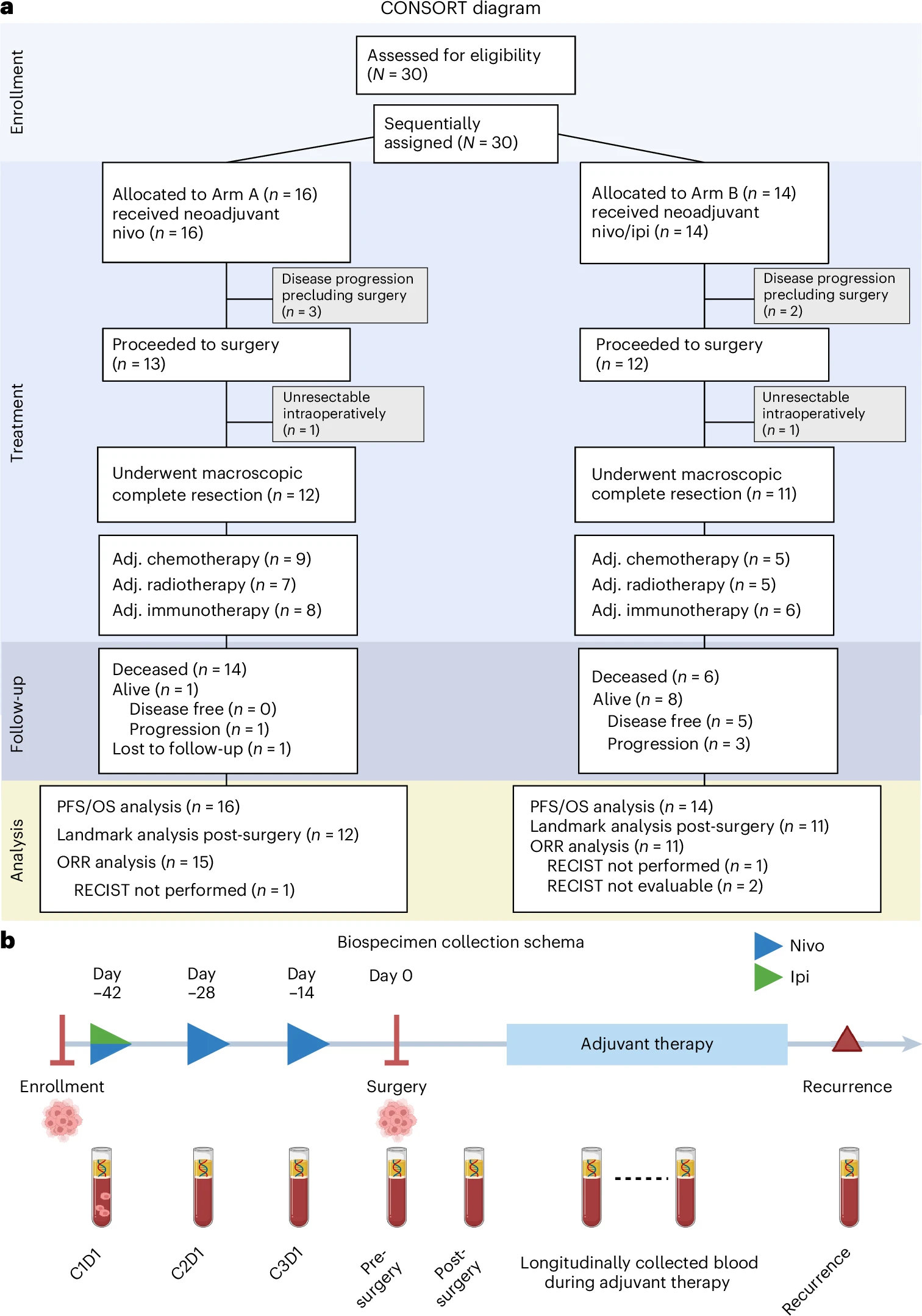
This was a prospective, multicenter, multiarm, non-comparative phase 2 trial (NCT03918252) evaluating perioperative immune checkpoint blockade in resectable diffuse pleural mesothelioma. Thirty adults with epithelioid or biphasic histology, ECOG performance status 0–1, and multidisciplinary confirmation of operability were enrolled sequentially to one of two neoadjuvant regimens.
In Arm A (n=16), patients received nivolumab 240 mg intravenously every two weeks for three cycles. In Arm B (n=14), patients received nivolumab 3 mg/kg every two weeks for three cycles with a single dose of ipilimumab 1 mg/kg on cycle 1, followed by surgery. After resection, both cohorts could receive platinum/pemetrexed chemotherapy and/or radiotherapy at the investigators’ discretion, and all patients were planned to receive adjuvant nivolumab 480 mg every four weeks for one year.
The co-primary endpoints were feasibility—defined as at least 80% of patients proceeding to surgery without a treatment-related delay beyond 24 days of the planned date—and safety, assessed by the incidence of dose-limiting toxicities during the neoadjuvant period. Secondary endpoints included radiographic response per mRECIST and the safety profile of adjuvant nivolumab. Key exploratory endpoints comprised progression-free and overall survival, alongside longitudinal circulating tumor DNA monitoring using a tumor-informed whole-genome-sequencing liquid biopsy to quantify residual disease and on-treatment molecular dynamics.
Results of the Study
- Arm A: 81.3% → surgery; Arm B: 85.7% → surgery.
- One DLT per arm (pneumonitis, ALT elevation).
- Grade ≥3 ICB-related adverse events: Arm A (18.8%), Arm B (7.1%).
Survival
- Arm A: Median PFS 9.6 mo; OS 19.3 mo.
- Arm B: Median PFS 19.8 mo; OS 28.6 mo.
Radiographic Response (mRECIST)
- Arm A ORR: 13.3%; Arm B ORR: 0%. Most patients had stable disease.
ctDNA Analysis
- Persistent ctDNA during neoadjuvant therapy predicted progression and failure to reach surgery (p=0.00013).
- Detectable ctDNA at cycle 3 or pre-surgery → significantly shorter PFS (p=0.027, p=0.0059).
- ≥95% reduction or clearance of ctDNA → longest PFS (median 23.3 mo).
- ctDNA dynamics often diverged from radiographic response, highlighting superior sensitivity.
Key Takeaway Messages
- Perioperative immunotherapy is feasible and safe in resectable DPM, with >80% of patients proceeding to surgery.
- Nivolumab + ipilimumab showed promising efficacy signals (median OS ~29 mo), despite small numbers.
- ctDNA residual disease tracking provided a highly sensitive molecular readout of treatment efficacy, outperforming imaging in predicting progression and recurrence.
- Persistent or rising ctDNA identified early treatment failure, while ctDNA clearance was strongly associated with durable benefit.
- Findings warrant larger, randomized studies to define the role of perioperative ICB and ctDNA monitoring in resectable mesothelioma.
Overview of Diffuse Pleural Mesothelioma
Diffuse pleural mesothelioma is a rare, asbestos-related cancer of the pleural lining, usually diagnosed at advanced stages and associated with poor prognosis. Standard care for unresectable disease now includes immune checkpoint inhibitors, which improve survival over chemotherapy.
The role of surgery remains controversial due to mixed evidence from randomized trials. Imaging assessment is challenging, as tumors spread in a diffuse sheet-like pattern rather than forming discrete nodules. Novel biomarkers such as ctDNA offer the potential to refine patient selection, monitor residual disease, and personalize treatment strategies.
You can read the full article here.
Written by Toma Oganezova, MD, Editor-in-Chief of OncoDaily IO