Immunotherapy has redefined modern oncology by harnessing the immune system to recognize and eliminate malignant cells, enabling durable disease control in settings where traditional cytotoxic approaches may offer limited long-term benefit. The pace of innovation remains high, with the U.S. FDA publishing annual and rolling updates of novel drug approvals and oncology regulatory activity, including approvals relevant to immuno-oncology and advanced biologic products.
Photo: Depositphotos
As immunotherapy expands, the delivery challenge is no longer limited to clinical decision-making it also includes preserving product integrity. Many immunotherapies are time- and temperature-sensitive pharmaceutical products (TTSPPs) whose quality can be degraded if they are stored or transported outside defined environmental conditions. The WHO’s TTSPP guidance outlines the core requirements for safe storage, temperature control, monitoring, documentation, and distribution across the supply chain.
This article explores how immunotherapy is transforming cancer care, why biologic complexity makes these therapies delicate, and how pharmacists and multidisciplinary teams safeguard quality from storage and cold chain logistics to bedside verification and safe administration while highlighting emerging technologies that strengthen traceability and stewardship.
How Immunotherapy Is Changing Cancer Care and Why Drug Safety Matters
Immunotherapy has fundamentally changed cancer care by modulating immune recognition and activation to produce durable antitumor responses. Approaches such as checkpoint inhibitors, cellular therapies, and antibody-based platforms have become integral in multiple tumor types. Alongside these clinical gains, however, immunotherapies introduce an added layer of complexity: the therapies themselves are often biologically intricate and environmentally sensitive.
This sensitivity matters because a therapy that is clinically “right” can still fail if its potency is compromised during storage, preparation, or administration. The WHO specifically defines TTSPPs as products that may degrade to the extent that they “no longer perform as originally intended” when not maintained within predefined conditions placing handling integrity on the same level of importance as prescribing accuracy.
Why Does the Complexity That Makes Immunotherapy Effective Also Make It So Delicate?
Immunotherapy products frequently include large biologic molecules (e.g., monoclonal antibodies), living cells (e.g., cellular therapies), or vector-based platforms, each of which can be destabilized by temperature excursions, light exposure, mechanical agitation, or inappropriate preparation. Unlike many small-molecule drugs, biologics may undergo structural changes (e.g., denaturation, aggregation) that reduce functional activity or alter immunogenicity.
Because many immunotherapies fall under TTSPP principles, their safe use depends on continuous control of environmental conditions and a rigorously documented chain of custody and temperature monitoring across storage and transport.
Optimal Storage and Monitoring: The Pharmacist’s Critical Role in Preserving Potency
Pharmacists are central to immunotherapy stewardship because they operationalize stability requirements into practice: validated storage conditions, continuous monitoring, documented responses to excursions, and staff training for correct handling.
WHO TTSPP guidance emphasizes documented procedures for storage and transport, including qualification of temperature-controlled areas, monitoring systems, and defined corrective actions when excursions occur.
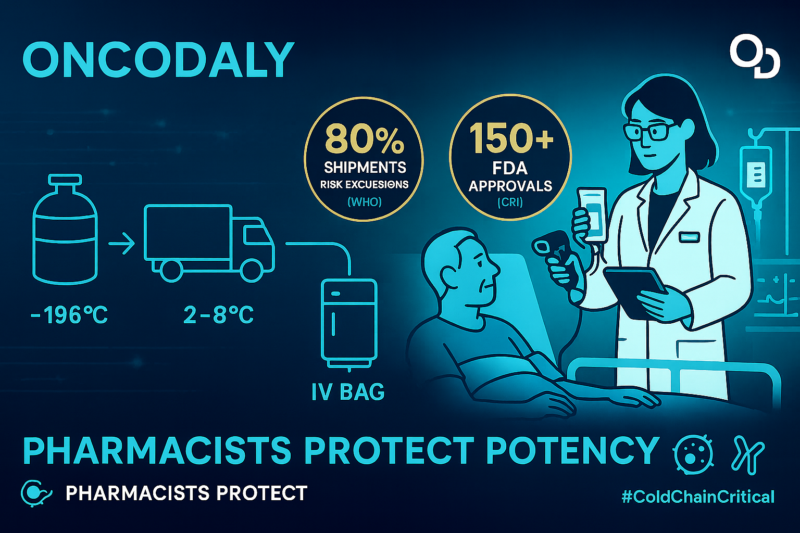
In practice, pharmacist-led safeguards often include: (1) continuous digital temperature logging with alarms, (2) routine calibration and equipment qualification, (3) inventory controls that ensure correct product rotation and expiration oversight, and (4) standardized preparation workflows that minimize avoidable stress to sensitive biologics. These steps reduce preventable loss of potency and strengthen patient safety.
Cold Chain Management and Transportation Challenges From Manufacturer to Pharmacy
Cold chain integrity is a foundational requirement for many immunotherapies, especially products requiring strict refrigeration, ultra-low temperatures, or cryogenic conditions. The WHO TTSPP framework describes core expectations for distribution systems handling temperature-sensitive medicines, including risk-based controls, temperature mapping and monitoring, documentation, and accountability across handoffs.
Every node manufacturer, shipper, receiving dock, pharmacy, and clinical unit—represents a potential failure point where delays or excursions can compromise product quality. As immunotherapy pipelines expand, strengthening cold chain infrastructure and training across the full delivery ecosystem becomes an essential patient safety priority, not merely a logistical one.
How Can Immunotherapy Be Safely Verified and Administered at the Patient’s Bedside?
The final safeguard is at the bedside. Verification and documentation practices ensure that upstream integrity is translated into correct patient care.
A core safety expectation is the use of at least two patient identifiers before administering medications. The Joint Commission outlines this requirement as part of its National Patient Safety Goals, emphasizing that identifiers may include name, assigned ID number, date of birth, and other person-specific identifiers (and should not rely on room number).
This aligns with WHO patient safety recommendations encouraging at least two identifiers to verify patient identity. cdn.who.int
Technology can further strengthen bedside safety. Barcode-enabled medication workflows can prevent many selection and administration errors when implemented correctly, and ISMP guidance supports barcode scanning as a key defense against wrong drug/strength/dosage form errors (while also warning against unsafe workarounds).
You Can Also Read 10 Must-Read Posts in Immuno-Oncology This Week by OncoDaily

What Emerging Technologies Are Shaping the Future of Immunotherapy Delivery?
The next phase of immunotherapy delivery will depend on integrating logistics reliability with clinical precision. Real-time monitoring, stronger documentation practices, and improved traceability across the TTSPP supply chain are increasingly supported by digital platforms and advanced analytics—provided they are embedded into multidisciplinary stewardship models led by pharmacists, oncologists, nurses, and supply chain teams.
In parallel, regulatory monitoring continues to evolve as new products enter oncology practice, with the FDA providing centralized access to approval pathways and annual/rolling approval reporting.
Ultimately, the full benefit of immunotherapy depends not only on discovery and clinical trial success, but also on healthcare systems’ ability to deliver these therapies with verified identity, maintained potency, documented integrity, and safe administration at every step.
Written by Eva Zeynalyan
FAQ
What is immunotherapy and how does it work in cancer treatment?
Immunotherapy is a cancer treatment that activates or enhances the body’s immune system to recognize and destroy cancer cells. Unlike chemotherapy, which directly kills rapidly dividing cells, immunotherapy targets immune pathways—such as immune checkpoints or tumor antigens—leading to more durable and targeted anti-tumor responses in many cancers.
Why is immunotherapy considered more sensitive than chemotherapy?
Most immunotherapies are biologic products, including monoclonal antibodies and living cells, which are highly sensitive to temperature, light, and handling conditions. Unlike small-molecule chemotherapy drugs, biologics can lose structure, potency, or safety if exposed to improper storage or transport conditions.
What does TTSPP mean in immunotherapy handling?
TTSPP stands for Time- and Temperature-Sensitive Pharmaceutical Products, a classification defined by the World Health Organization. Many immunotherapies fall under this category, meaning their quality and effectiveness depend on strict control of temperature and handling throughout storage, transport, and administration.
Why is temperature control critical for immunotherapy drugs?
Temperature excursions can cause biologic immunotherapy drugs to degrade, aggregate, or lose biological activity. Even brief exposure outside recommended ranges may reduce treatment effectiveness or increase the risk of adverse reactions, making continuous temperature monitoring essential.
What happens if immunotherapy drugs are not stored properly?
Improper storage can lead to reduced potency, loss of therapeutic effect, or compromised safety. In some cases, affected products must be discarded, resulting in treatment delays, increased healthcare costs, and potential harm to patients if compromised drugs are administered.
What is the pharmacist’s role in immunotherapy safety and delivery?
Pharmacists play a critical role in safeguarding immunotherapy by ensuring correct storage conditions, monitoring temperature excursions, overseeing preparation, verifying documentation, and educating healthcare staff. Their stewardship helps preserve drug integrity and protect patient safety across the entire care pathway.
How is immunotherapy verified before administration to a patient?
Before administration, immunotherapy undergoes multiple verification steps, including confirmation of the correct drug, dose, patient, and timing. This often involves pharmacist review, nursing double-checks, barcode scanning, and comparison with electronic medication orders to prevent errors.
Why are two patient identifiers required before immunotherapy administration?
Using at least two patient identifiers—such as name and date of birth—is a key safety standard recommended by the WHO and The Joint Commission. This practice reduces the risk of administering immunotherapy to the wrong patient, which can have serious or fatal consequences.
10. How can healthcare systems prevent errors in immunotherapy administration?
Preventing errors requires a multidisciplinary approach that includes pharmacist oversight, standardized protocols, staff training, barcode medication administration, double verification, and continuous quality monitoring. Integrating technology with teamwork is essential to ensure immunotherapy is delivered safely and effectively.


