This week in OncoDaily Immuno-Oncology, new studies are advancing precision immunotherapy — from neoadjuvant intralesional therapies in melanoma and germline variants affecting urinary tract cancer ICI response, to TNBC predictors, multi-omics insights in NSCLC, and ROR2-targeted CAR-T strategies. Updates also include dual checkpoint blockade, B cell–driven glioblastoma immunity, and PD-L1 resistance mechanisms, highlighting bold strides in next-generation IO.
Marco Donia (Professor at University of Copenhagen):
“Neoadjuvant intralesional immunocytokines for macroscopic, resectable stage ≥III melanoma
(Annals of Oncology, Kähler et al., October 2025)
Neo-adjuvant immunotherapy with checkpoint inhibitors is a new standard-of-care for patients with macroscopic, resectable stage ≥III melanoma (more at). Is there a role for intralesional therapies?
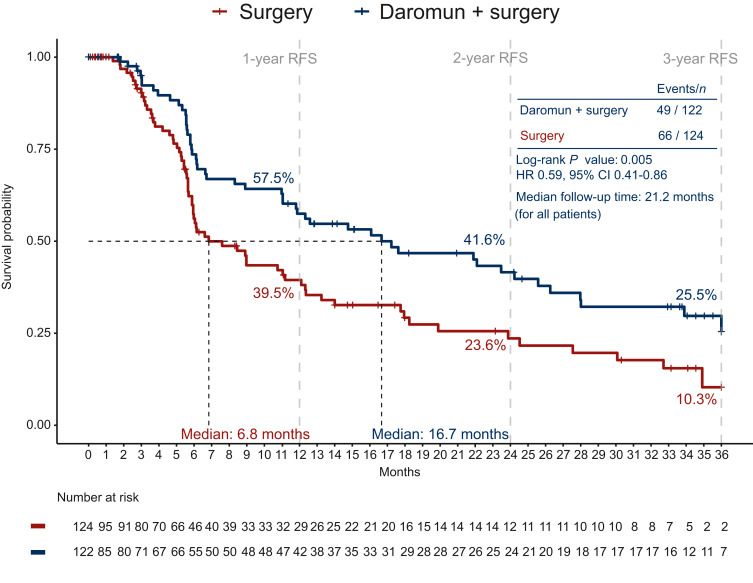
Phase III PIVOTAL trial: intralesional daromun (L19IL2 + L19TNF) weekly for 4 weeks before surgery vs. upfront surgery in resectable stage ≥III melanoma
Key points
- 246 patients enrolled, most heavily pretreated (74% ≥2 prior surgeries; 35% prior systemic therapy – meaning including a patient group with recurrence after prior anti-PD-1).
- Recurrence-free survival (RFS): median 16.7 months with daromun vs. 6.8 months with surgery alone (HR 0.59; P=0.005);
- Distant-metastasis free survival (DMFS): median 28 months with daromun vs 17.7 with surgery alone (HR 0.60, P = 0.029).
- Safety: 26.3% Treatment-related grade ≥3 adverse events (11% were injection-site reactions)
Take-home messages
- Neoadjuvant daromun is safe and has activity (RFS and DMFS improvement), even in a high-risk, pretreated population.
Clinical implications
- Immune checkpoint inhibitors remain the current standard of care
- Is there a potential space for Daromun/intralesional therapies in the neo-adjuvant space? Perhaps for patients with locoregional recurrence after prior anti-PD-1 therapy, or not eligible for checkpoint inhibitors (however, the study did not formally test this)
For other intralesional neo-adjuvant therapies.
n.b. while criteria for determining a “recurrence event” are different in neo-adjuvant trials and only ~1/3 of the patients in this study received adjuvant therapy, the 1-year RFS in the control group of PIVOTAL had a worse RFS (39%) compared to control groups of trials of adjuvant and neo-adjuvant therapy (NADINA: 57%; SWOG S1801 ~60%, potentially reflecting a population with worse baseline features
Congratulations to the authors for this important study!”
Jad Chahoud (Associate Member, Department of Genitourinary Oncology and Medical Director of IPOP at Moffitt Cancer Center):
“New Publication Alert – Journal for ImmunoTherapy of Cancer (JITC)
Thrilled to share a huge team effort exploring the germline genetics of urinary tract cancers (UTCs) and their impact on immune checkpoint inhibitor (ICI) response.
Key findings:
- Through whole-exome sequencing of 810 UTC patients, we identified FOXP3-related germline variants in ~16% of patients.
- These variants confer susceptibility to UTC and interact with DNA mismatch repair pathways.
- Patients carrying these variants had decreased tumor FOXP3 expression, a colder tumor microenvironment, and significantly worse outcomes following ICI.
Our results highlight germline-tumor interactions as an underappreciated factor in both cancer susceptibility and immunotherapy resistance.
Read the full article here below.”
Nour Abuhadra (Assistant Professor at Memorial Sloan Kettering Cancer Center):
“Thrilled to share our latest: the largest prospective study on predictors of response to neoadjuvant KN-522 in metaplastic TNBC. Long overdue for MpBC patients to have biology-driven data!”
Arutha Kulasinghe (Associate Professor and Principal Research Fellow at The University of Queensland):
“We are super proud of our Yale School of Medicine and Clinical-oMx Lab UQ News team for our new publication in Nature Genetics titled ‘Spatial signatures for predicting immunotherapy outcomes using multi-omics in non-small cell lung cancer’. Let’s get into it…”
Mohamad Mohty (Professor of Hematology at Sorbonne University):
“CAR-T and bispecifics are powerful, life-prolonging tools, but their success depends heavily on timing, disease context, and the ability to debulk beforehand. The field truly needs innovation beyond the current immunotherapy paradigm.”
William Curry (Chief Medical Officer at Massachusetts General Hospital / MGPO):
“Intratumoral B cell and interferon signatures in newly diagnosed glioblastoma are associated with longer survival in patients treated with SurVaxM | Cancer Immunology, Immunotherapy… Don’t forget the B cells! Need coordinated immunity to slow GBM.”
John Gordon (co-Founder, Director, VP Scientific Affairs at Celentyx Ltd):
“Cancer | Immune Checkpoint Inhibition | ICB | Overcoming Resistance to anti-PDL1 Immunotherapy: Mechanisms, Combination Strategies and Future Directions | ‘Irresistible’ OPEN ACCESS Review Breaking Online | Tcells |
Cancer cells express high levels of programmed cell death-ligand 1 (PD-L1) to evade immune surveillance. PD-L1 interacts with PD-1 on T cells to make them non-functional. Thus, PD-L1 and PD-1 are pivotal targets in cancer immunotherapy. While anti-PD-1/PD-L1 therapies have offered renewed hope for many patients, their modest efficacy remains a critical concern. This underscores the urgent need to unravel the intricate mechanisms that govern both therapeutic responses as well as resistance to immunotherapy.
Here, Kartik Mandal, Ganesh Kumar Barik, and Manas Santra explore the multifaceted nature of PD-L1, including factors that regulate its expression, tumor-immune interactions, and the resistance mechanisms associated with anti-PD-L1 immunotherapy. Several promising strategies have been explored to overcome these challenges, such as combination therapies, modulation of the tumor microenvironment, neoantigen targeting, and dynamic biomarker monitoring.
Outcomes of these approaches, integrating advanced technologies like high-resolution imaging, machine learning, multi-omics profiling, and liquid biopsy for soluble PD-L1 detection emerge as a powerful means to refine patient stratification.
Together, these innovations pave the way toward more precise and personalized immunotherapy, maximizing clinical benefits for cancer patients. Additionally, the evolving landscape of clinical trials involving anti-PD-1/PD-L1 monoclonal antibodies has been explored, emphasizing the integration of immune checkpoint therapies with chemotherapy, radiotherapy, targeted therapy, CAR-T, and metabolic immunotherapy to overcome resistance in refractory cancers.
By embarking on these challenges and leveraging novel therapeutic strategies, this review intends to advance the understanding of more effective, personalized cancer immunotherapies, ultimately improving outcomes for a broader range of patients.”
Michael Hudecek (Lead – Würzburg Branch Site Cellular Immunotherapy at Fraunhofer IZI):
“ROR2 as a promising target for cellular immunotherapy in cancer
We identified the receptor tyrosine kinase-like orphan receptor 2 (ROR2), which is upregulated and associated in many types of cancer and linked to a worse therapeutic prognosis, as a promising target for cellular immunotherapy.
In our study, just published in Cell Reports Medicine by Cell Press, we analyzed public gene expression data showing that higher ROR2 levels are associated with poorer survival in six types of cancer, including low-grade glioma, thyroid, stomach, bladder, and several classes of kidney cancers.
ROR2 is also consistently expressed in multiple myeloma and we developed ROR2-CAR-T cells that effectively fight MM tumors in animal models, and are still effective after BCMA antigen loss.
ROR2-CAR-T cells also worked effectively against kidney cancer in in vitro and in vivo studies, with no signs of harming healthy tissues, supporting ROR2 as a safe and promising target for CAR-T therapy.
Our results informed the initiation of a first-in-human clinical trial with ROR2-CAR-T cells which is going to commence in early 2026.
Publication title: ROR2-specific CAR T cells are effective against hematologic and solid tumors, and well tolerated in mice”

Kristina Jankovic (Medical Oncology Resident at University Clinical Center Nis):
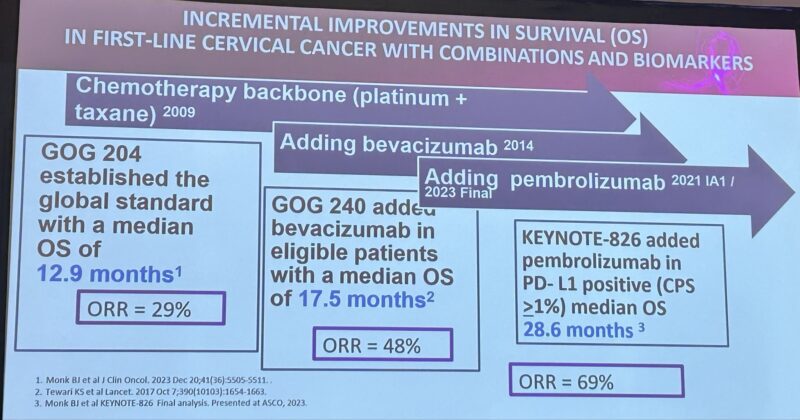
“Immunotherapy in GyneOnc Standard 1L Cervical Cancer : KEYNOTE-826 added pembrolizumab in PD-L1+ (CPS>1%) patients → median OS 28.6 mo, ORR 69%. Bevacizumab adds extra benefit when not contraindicated.”
Pashtoon Kasi (Medical Director at City of Hope):
‘Hidden spark: From cold to hot in BRAF colorectal cancer’
Debunking the myth: Immunotherapy does not work for MSS colorectal cancer.
My perspective on Van Morris and Scott Kopetz work Cancer Cell.
50 day.”
Written by Rima Grigoryan, MDc, Assistant Editor of OncoDaily IO.


