At OncoDaily Immuno-Oncology, we spotlight this week’s defining voices – from CRISPR 2.0–engineered T cells and NK–MSC conjugates with IL-15, to IL-9 “private line” signaling and the first CAR T success in ulcerative colitis. Add in insights on NSCLC resistance tailoring, CheckMate 9ER in RCC, biopharma’s 2025 watchlist, and microbiome–IO links, and you have the science and strategy steering immunotherapy forward.
Michael Hudecek (Lead of Würzburg Branch Site Cellular Immunotherapy at Fraunhofer IZI):
“I firmly believe that the next generation of clinically effective CAR- and TCR-modified T cell immunotherapies will also be gene-engineered to enhance both efficacy and safety.
CRISPR 2.0 is more powerful and precise than conventional CRISPR. Rather than inducing double-strand breaks in DNA, base and prime editing enable the targeted and efficient rewriting of gene sequences. This technology holds the potential to enhance the effectiveness of T cells against tumors.
In our latest review, we highlight both the challenges and the promising opportunities that come with implementing CRISPR 2.0 in T cell therapy.
Publication: Next-generation T cell immunotherapies engineered with CRISPR base and prime editing: challenges and opportunities
Thanks to first author Karl Petri and the co-authors Elvira D’Ippolito, Annette Künkele, Ulrike Köhl, Dirk H. Busch and Hermann Einsele.”

Roberto Ferrara (Medical Oncology Researcher at Vita-Salute San Raffaele University):
“It is time to tailor 1st line treat in NSCLC according to primary/acquired ICI resistance. Our reconstructed IPD analysis (19 RCTs) shows that adding platinum chemotherapy to ICI may acquired resistance (+15%) vs mono-ICI and duration of response (5-7 mo)”

Michel Frank Ferrazo (Clinical Research Analyst – Technical Training-3 at Hemostasis Laboratory – Unicamp):
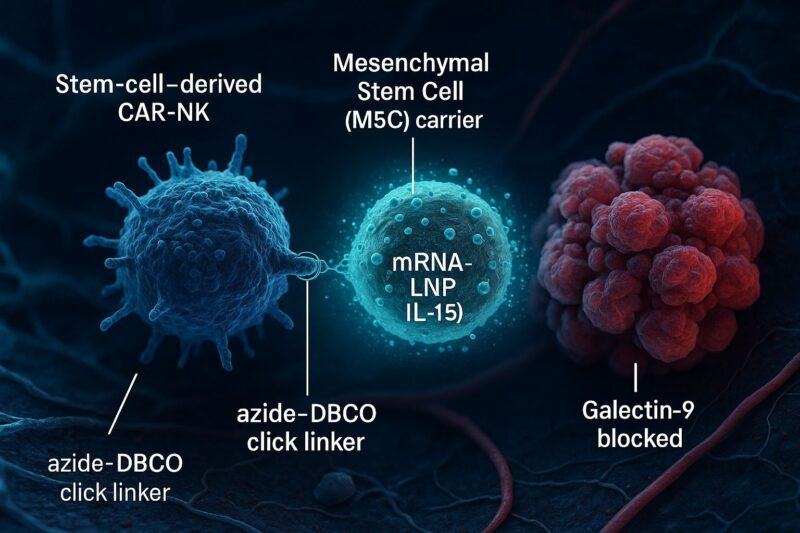
“Smart NK-cell delivery meets local cytokine support + checkpoint blockade — a modular strategy for solid tumors just published in Advanced Science.
What’s the big idea?
- Bioorthogonally “clicked” MSC–NK conjugates (azide ↔ DBCO)
- mRNA–LNP–armed adipose MSCs to secrete IL-15 locally
- Synergy with Galectin-9 (Gal-9) blockade to reverse NK dysfunction
Why it matters
- Better homing: MSCs are tumor-tropic; bringing NK cells along boosts intratumoral delivery.
- NK fitness on site: Transient, local IL-15 sustains cytotoxicity without systemic cytokine baggage.
- Checkpoint angle: Targeting Gal-9/TIM-3 helps rescue exhausted NK cells in the tumor microenvironment (TME).
What the data show (preclinical)
- Orthotopic lung and ovarian PDX models: conjugates infiltrate more, hit harder, and pair well with anti-Gal-9.
- Clear evidence of specific cell–cell conjugation and enhanced NK function when IL-15 is supplied locally.
What’s novel
- Click chemistry to physically couple therapeutic cells (no extra genome engineering).
- mRNA–LNP turns MSCs into time-limited IL-15 bioreactors, aligning with NK biology.
- Triple modality: targeted delivery + cytokine support + checkpoint inhibition
Caveats to watch
- First-pass lung trapping of MSCs after IV infusion—needs rigorous biodistribution kinetics.
- MSC duality in TME—demand robust safety readouts (PK, serum cytokines, histopath).
- Models (PDX/immunodeficient) may underrepresent full immune crosstalk.
- CMC complexity: bi-cellular product ⇒ specs for % conjugation, binding stability, potency assays, cryo-viability.
What I’d test next
- Humanized or immunocompetent models to map broader immune effects.
- Head-to-head vs CAR-NK mbIL-15 and vs “loose” co-infusion (no click) to isolate the true delta of conjugation.
- Administration routes (regional/intra-arterial) to mitigate pulmonary sequestration.
- Expand checkpoints beyond Gal-9 (e.g., TIGIT, Siglec-7/9).
- iPSC-NK or standardized allo-NKs for scalability.
Bottom line
This is a compelling framework for NK therapy in solid tumors: carrier cell for targeting, programmed local cytokine, and checkpoint relief—all modular and, in principle, tunable. Lots to like, and plenty to validate.”
Nicholas P Restifo (Chief Medical Officer at Medici Therapeutics):
“T Cells a Private Line: Rewriting Cell Therapy with IL-9/IL-9R
Instead of shouting at the whole immune system, imagine whispering to only the tumor-killing cells. That’s the idea: a private, non-cross-reactive cytokine signal that boosts the right T cells without collateral chaos. (If you remember “orthogonal” from geometry, think independent here.)
At the forefront are Anusha Kalbasi and Chris Garcia. In two recent papers they move from synthetic orthogonality to native near orthogonality in engineered T cells.
Then – Nature 2022:
A synthetic o9R switch (oIL-2Rβ-ECD + IL-9R-ICD) lets an orthogonal IL-2 variant drive a STAT1/3/5 program. Result: Tscm-like persistence with effector punch and strong tumor control in mice – even without lymphodepletion (preclinical). (We discussed this in a Kishton & Restifo commentary.)
Now – bioRxiv 2025:
Leverage biology’s selectivity: engineer T cells with wild-type IL-9R and dose IL-9. Because IL-9R is scarce in normal tissues, IL-9 acts like a private line to engineered cells.
Versus o9R, IL-9R + IL-9 shows stronger JAK/STAT, adds STAT4 (IL-12-like), deeper infiltration, more stemness, and better tumor control – with repeat IL-9 pulses → repeat expansion peaks, and efficacy at lower cell doses (preclinical, incl. human CAR-T in NSG). They used a mouse-albumin–IL-9 fusion to extend half-life (a human-albumin version is the translational path), though anti-drug antibodies (ADA) remain a watch-item.
It’s the STAT recipe, not “more signal.”
There’s a Goldilocks zone: both attenuation and over-amplification of proximal IL-9R signaling hurt efficacy. STAT1 is the rheostat – low pSTAT1 → proliferative/stem-like; high pSTAT1 → terminal/dysfunctional. Best outcomes sit mid-pSTAT1 with a balanced STAT1/3/4/5 mix.
Why this is important
- A druggable, outpatient-friendly control knob for TIL/NeoPBL/CAR-T that may enable no/low-LD regimens.
- Cleaner, selective expansion of the cells that do the work.
Caveats (the human reality):
- Safety first: IL-9 in humans is unproven; ADA is possible, and IL-9R appears in lung, a tough combo given TIL/IL-2-like capillary-leak risk.
- No-LD reality check: intact immunity can mean poor engraftment, innate clearance, and bystander IL-9R upregulation in inflamed tissue.
- Scale gap: what blooms at 0.5M cells in mice can sputter in a 90-kg adult on steroids with scarred marrow and comorbid lungs.
- Finding Goldilocks isn’t obvious: use step-up IL-9 dosing with hard PD gates (infused-clonotype expansion, ctDNA drop, pSTAT signatures) before escalating.
Bottom line: the field is shifting from more cells to smarter, younger, more controllable cells.
Onward.”
Swapnil Narkhede (Sr officer at Indian Immunologicals Limited):
“Global Biopharma Watchlist 2025
The race is heating up across vaccines and biosimilars – reshaping healthcare access, pricing, and strategy.
Current Molecules & Moves Driving Disruption:
- Keytruda Qlex (SC pembrolizumab) → FDA approval boosts lifecycle defense.
- Stelara (ustekinumab) biosimilars → Selarsdi, Otulfi, Starjemza gain interchangeability.
- Denosumab biosimilars (Prolia/Xgeva class) → FDA & EMA green lights (Bildyos, Bilprevda, Jubereq, Osvyrti, etc.).
- Insulin Aspart “Kirsty” → Interchangeable biosimilar to Novolog.
- Sanofi–Vicebio deal → RSV vaccine with molecular clamp tech.
- BioNTech–CureVac merger → Strengthens mRNA and oncology vaccine pipelines.
- Dr. Reddy’s + Alvotech → Keytruda biosimilar co-development.
Competitive Intelligence takeaway:
- Companies defending blockbuster biologics with new formulations.
- Biosimilars gaining interchangeability = faster market penetration.
- Vaccine platforms diversifying beyond mRNA.
- Partnerships in India & EU set new global benchmarks.
The next 12 months will define who leads, who follows, and who exits.
Question for my network → Which molecule do you think will disrupt the market most in 2026?”
Yan Leyfman (Medical Oncologist, Co-Founder and Executive Director of MedNews Week):
“Groundbreaking in UC!
For the first time, CAR T-cell therapy — originally built for lymphoma and now used in lupus & systemic sclerosis — has shown success in ulcerative colitis (UC).
Case details:
- 21-year-old with severe, multidrug-resistant UC (failed steroids, biologics, JAK inhibitors, cyclosporine, and more)
- Received CD19 CAR T-cell therapy after multiple failed options including blinatumomab.
- Achieved complete clinical, biochemical, and mucosal remission within 14 weeks.
- Gained 20 lbs and resumed normal life.
- Only mild side effects (grade 1 CRS, neutropenia).
Why this matters:
UC has never been considered a B-cell–driven disease, making this remission unexpected. If replicated, CAR T may expand its reach far beyond current autoimmune indications.
Caveat: Just 1 patient, short follow-up (14 weeks). Larger trials needed.
Still – a major step that could reshape UC treatment in the future.”

Sarah Scagliarini (Medical Oncologist at AORN Cardarelli in Naples):
“Grand finale for Nivolumab + Cabozantinib in 1 line for mccRCC!
Checkmate 9ER final analysis in press on Annals of Oncology!”
Arsela Prelaj (Medical Oncologist and PhD in Bioengineering at IRCCS Foundation National Cancer Institute):
“Today at Fondazione IRCCS Istituto Nazionale dei Tumori we had the opportunity to attend the presentation of projects funded under the Lines of Research grants, with a special focus on cancer immunotherapy.
This internal meeting brought together clinicians and researchers from across disciplines to discuss innovative ideas, early results, and translational perspectives aimed at advancing cancer care.
The program featured exciting talks on:
- Tracing anti-cancer immunity in patients undergoing liver transplantation for HCC after immunotherapy Licia Rivoltini @sherie boorie
- The role of ZEB1 in suppressive neutrophils and neutrophil extracellular traps in non-small cell lung cancer Marta Brambilla claudia proto
- Roadmap for successful clinical development of bispecific antibodies and CAR-T therapies Mariangela Figini
- Interplay between tumor genotypes, immune environment, and CAR T-cell failure in large B-cell lymphoma Giada Zanirato Cristiana Carniti
- Translational correlates of response to anti-PD1/anti-EGFR in cutaneous squamous cell carcinoma using ex-vivo 3D platforms Viviana Salvatore Alfieri
- Discovery of biomarkers for immune checkpoint blockade in pleural mesothelioma
Bladder Cancer and Non invasive Understanding tumor and immune dynamics and predicting poor outcome to intravescical therapies (beg) in patients with non-muscle-invasive bladder cancer (nmibc) that had progressed in locally/advanced metastatic disease Patrizia Giannatempo
These sessions not only highlighted cutting-edge science but also fostered collaboration within INT, creating fertile ground for the development of future projects.
It was inspiring to see how our institute continues to invest in internal research lines to strengthen innovation, empower young investigators, and accelerate translation into patient benefit within academic no profit grants. A great thank to our Scientific Directorate at @istituto and Ministero della Salute Fondazione IRCCS Istituto Nazionale dei Tumori di Milano.”

Siow Ming Lee (Professor of Medical Oncology at University College London Hospitals, UK):
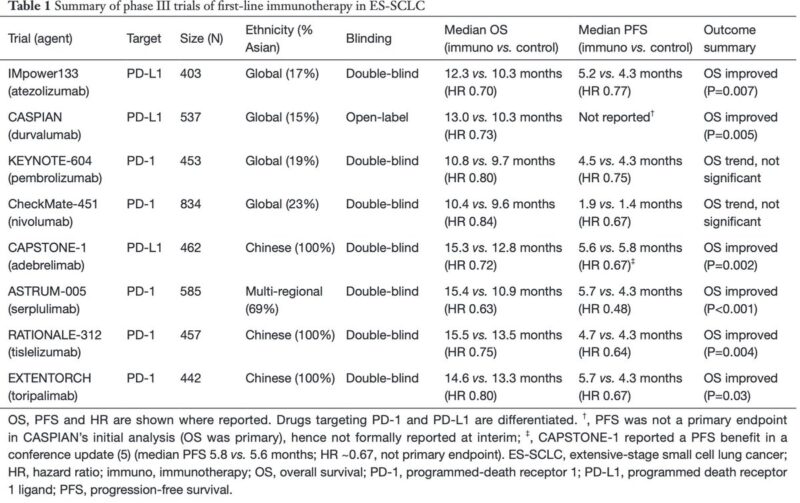
“ES-SCLC: charting the future of immunotherapy. Our commentary on toripalimab (EXTENTORCH) in the global trial landscape: OS benefit in Chinese trials Need for global validation Role of HLA & biomarkers in future trials.
Emile Voest (Professor of Medical Oncology at The Netherlands Cancer Institute):
“Very proud to share our work showing how the gut microbiome produces metabolites to influence immune cells and the outcome of immunotherapy in cancer patients. Exciting field to improve treatment outcome for future patients.”
Written by Rima Grigoryan MDc. Assistant Editor of OncoDaily IO.


