This week in OncoDaily Immuno-Oncology, ESMO25 delivered landmark advances – from KEYNOTE-B96 establishing the first immunotherapy survival benefit in platinum-resistant ovarian cancer, to RC48-C016 redefining outcomes in HER2-expressing urothelial cancer. Highlights included breakthroughs in ctDNA utility, ADC toxicity management, neoadjuvant IO superiority in colorectal cancer, and rare cancer wins with liver-targeted chemo-immunotherapy, marking a pivotal moment for next-generation precision IO.
Tanja Obradovic (Oncology Medical Strategy Advisor at Mercurial AI):
“February 20th next year is a date when great news for patients with platinum-resistant recurrent ovarian cancer can come as Merck announces that time for the FDA Prescription Drug User Fee Act (PDUFA) decision on impressive results of Phase III KEYNOTE-B96, also known as ENGOT-ov65 trial.
Trial has been just presented at ESMO2025 Presidential Symposium where Keytruda (Pembrolizumab) plus chemotherapy with or without bevacizumab delivered a statistically significant and clinically meaningful improvement in progression-free survival (PFS) and overall survival (OS) vs chemo with or without bevacizumab in patients whose tumors express PD-L1 (CPS ≥1).
This Keytruda combination will be the first immunotherapy demonstrating improved survival in this patient population that currently have very large medical need. Congratulations to Merck Women’s Cancer team and outstanding expert, innovative leader and trailblazer in bringing new therapies to patients Gursel Aktan!”
Paolo Tarantino (2025 Yvonne’s “Top Voice” Award Winner, Research Fellow at Dana-Farber Cancer Institute):
“Grateful for the chance to chair the ESMO25 ADC educational session and present on ADC toxicities.
With the use of ADCs rapidly expanding across cancers, it’s critical to be aware of how to identify, prevent and mitigate their side effects.
For an in-depth dive into the topic, read our Nature Review article.”

“ICYMI: At our October Stakeholder Webcast, Dr. Michael Gordon shared new pan-tumor data from more than 400 patients with refractory disease presented at ESMO2025 showing how BOT + BAL are challenging long-held limits in immuno-oncology.
Watch the full discussion and data highlights on demand.”

Petros Grivas (Clinical Director of the GU Cancer Program at the University of Washington):
“Indeed phenomenal broad impact by our friend Tom Powles including excellent presentation at ESMO25 evaluating the clinical utility of ctDNA in IO-naive patients with MIBC in adjuvant setting:
IMvigor011 validated hypothesis generated by IMvigor010.”

Kevin Harrington (Professor in Biological Cancer Therapies at Institute of Cancer Research):
“Having just returned from ESMO, it is a good opportunity to reflect on some important events over the last few days.
On Friday 17th October, I had the pleasure of co-chairing a session with Prof Irene Brana on Refining Strategies to Boost Immunotherapeutic Effectiveness in Head and Neck Cancer.
The session included superb presentations from Professor Inge Tinhofer-Keilholz (on organoid models for H&N cancer research) and Prof Makoto Tahara (on the rationale for combination therapies). I was able to present thoughts on the role of DNA repair inhibition, cell death modulation and innate immune activation via the STING pathway.
It was a very interactive and instructive session with lots of excellent take home messages for attendees. I hope it will also be viewed on the ESMO platform by those unable to attend.”

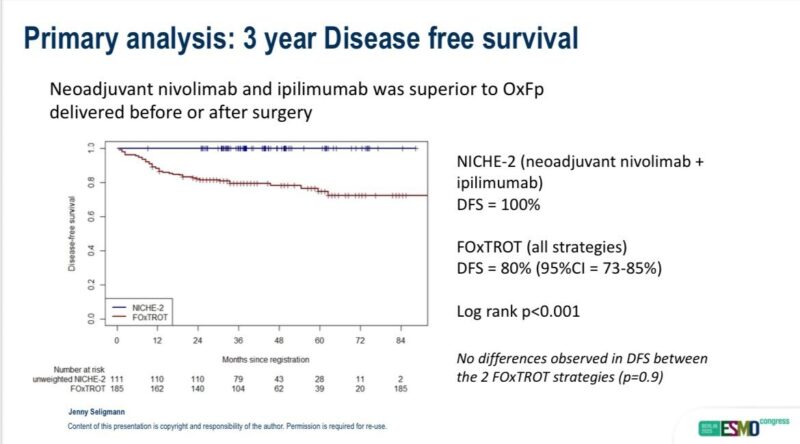
Jenny Seligmann (Professor of Gastrointestinal Oncology at University of Leeds):
“Proud to present combined NICHE2 + FOXTROT analysis at ESMO25
In summary
IO superior to chemo (pre or post surgery)
Little role of chemo in dMMR CC (!ATOMIC)
CT staging identified high risk patient
20% recurrence in “good prognosis” patients relevant with effective rx!
Further data to support neoadjuvant IO for routine use! ESP for T4 patients
Privilege to work with super NKI team.”
Petros Grivas (Clinical Director of the GU Cancer Program at the University of Washington):
“Congrats Shilpa Gupta for all your leadership, hard work, excellent discussion at ESMO25.
It was great to see you! Happy Diwali!”

Jacek Madeja (Founder at H2R Sp. z o.o.):
“A rare win in a rare cancer: Combining Delcath’s liver-targeted chemo (HEPZATO) with immunotherapy (ipi + nivo) helped 55% of patients stay cancer-free for a year vs 16% with chemo alone.
New hope for metastatic uveal melanoma.”
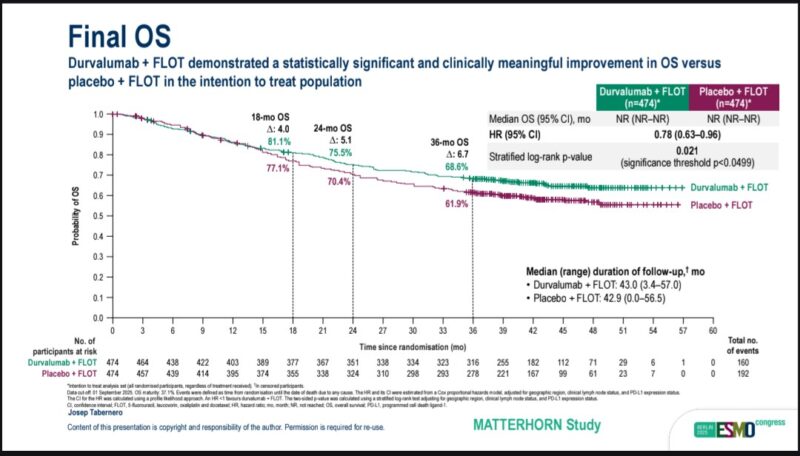
Lizzy Smyth (Clinical Lead at OCTO: Oxford Clinical Trials Office):
“We have seen the mountain top! MATTERHORN OS
ESMO25 OS continues to over time (~7% 3y) Benefit independent of PD-L1
Enough now to adopt as SOC?”
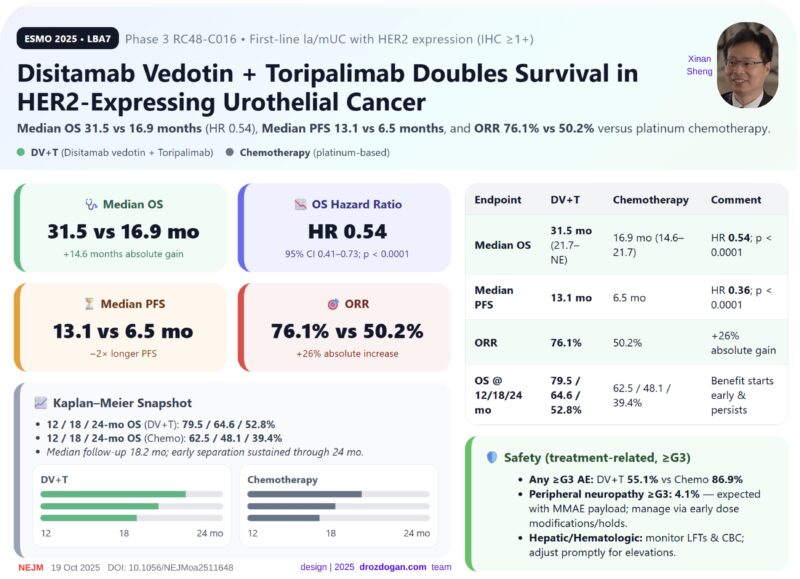
Mustafa Özdoğan (Head, Division of Medical Oncology at Memorial Healthcare Group):
“A standing ovation moment at ESMO25
In front of more than 9,000 cancer specialists and researchers, the Phase 3 RC48-C016 trial redefined the outlook for HER2-expressing urothelial cancer:
Disitamab vedotin + Toripalimab nearly doubled overall survival over chemotherapy – marking the arrival of the ADC + IO era in this disease.”
Written by Rima Grigoryan, MDc, Assistant Editor at OncoDaily IO.


