Dual blockade of the PD-1/PD-L1 axis and the VEGF/VEGFR pathway is one of the most clinically validated strategies to overcome immune evasion in solid tumors, particularly by reversing VEGF-driven immunosuppression (impaired dendritic cell maturation, increased Tregs, reduced T-cell trafficking) while simultaneously restoring cytotoxic T-cell activity. The clinical success of PD-(L)1 + anti-VEGF combinations (e.g., atezolizumab + bevacizumab in multiple settings) supports the biology, but combination therapy increases complexity, cost, and potential additive toxicity.
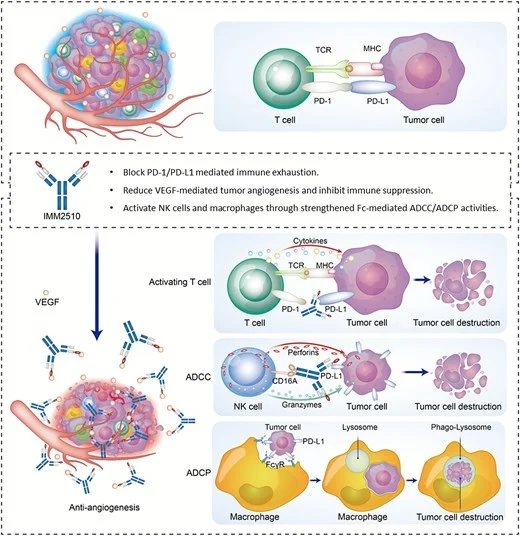
IMM2510 is positioned as a next-generation solution: a PD-L1 × VEGF bispecific antibody designed to deliver checkpoint blockade and angiogenesis inhibition in one molecule—plus an Fc engineered for enhanced ADCC, aiming not only to block PD-L1 signaling but also to actively eliminate PD-L1–expressing tumor and stromal cells.
Study Design and Methods
This preclinical study evaluated the PD-L1 × VEGF bispecific antibody IMM2510 (published January 15, 2026) across molecular, cellular, and in vivo systems. Binding and kinetic properties were assessed using ELISA, surface plasmon resonance, and flow cytometry, while functional blockade of PD-1/PD-L1 and VEGF/VEGFR signaling was examined through reporter and ligand-blocking assays. Anti-angiogenic activity was measured via HUVEC proliferation, and immune activation was analyzed using mixed lymphocyte reactions with interferon-γ release. Fc-mediated effector function was characterized by NK-cell–driven ADCC and macrophage-mediated ADCP. Antitumor efficacy was further tested in vivo using MC38-hPD-L1 syngeneic tumors in hPD-1 transgenic mice, as well as HCC827 NSCLC and MDA-MB-231 triple-negative breast cancer xenograft models.
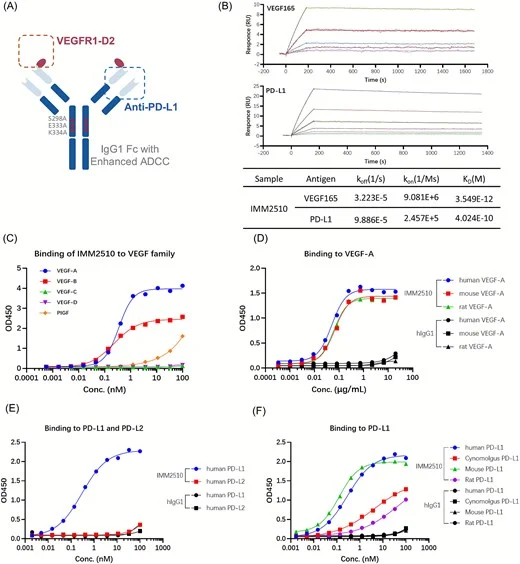
Results
Dual high-affinity target engagement and pathway blockade
IMM2510 binds PD-L1 and multiple VEGF-family ligands (VEGF-A, VEGF-B, PlGF) with high affinity and blocks:
- PD-1/PD-L1 interaction (restores TCR/NFAT signaling in reporter assays)
- VEGF/VEGFR signaling (suppresses VEGF-driven reporter activation and endothelial proliferation)
Immune activation + anti-angiogenic activity in functional systems
- Restored PD-1–suppressed signaling and increased IFN-γ in mixed lymphocyte reaction assays
- Inhibited VEGF-driven HUVEC proliferation, consistent with anti-angiogenic function
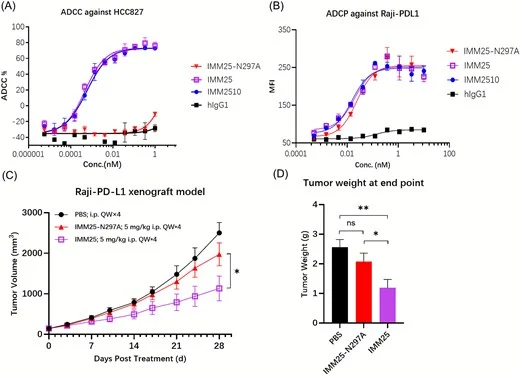
A major differentiator: Fc-engineered cytotoxicity (ADCC/ADCP)
Unlike many PD-(L)1×VEGF bispecific formats designed to be Fc-silent, IMM2510 incorporates Fc engineering to enhance FcγRIIIa binding and immune effector activity:
- Demonstrated potent NK-mediated ADCC against PD-L1+ tumor cells
- Demonstrated ADCP, supporting macrophage-mediated clearance
- In vivo comparison showed stronger antitumor activity when Fc function was intact vs Fc-silenced control antibody (supporting Fc contribution to efficacy)
Cooperative binding: VEGF165 enhances PD-L1 engagement
A particularly interesting mechanistic feature: preincubation with VEGF165 enhanced PD-L1 binding and checkpoint blockade activity, suggesting VEGF-rich microenvironments could amplify functional target engagement.
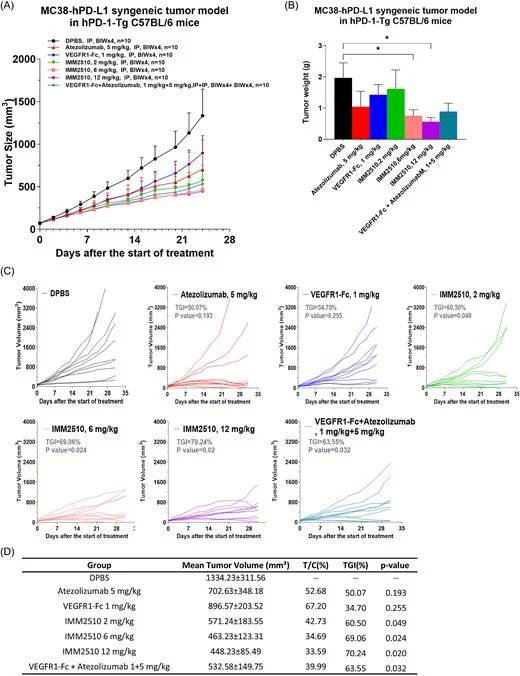
In vivo antitumor efficacy across models (including NSCLC)
IMM2510 induced dose-dependent tumor growth inhibition and showed superior activity compared with parental monotherapies and their combination in multiple models, including:
- Syngeneic immunocompetent setting (MC38-hPD-L1 in hPD-1 Tg mice)
- Human NSCLC xenograft (HCC827) with tumor regression in a subset
Why IMM2510 Represents More Than Another PD-(L)1 + VEGF Strategy
IMM2510 should not be interpreted simply as an additional dual-target checkpoint–angiogenesis program. Instead, it represents a three-layer therapeutic architecture integrated within a single molecule.
First, PD-L1 checkpoint blockade restores antitumor immunity by reinvigorating exhausted T cells and reactivating immune surveillance.
Second, VEGF ligand trapping simultaneously suppresses angiogenesis and mitigates VEGF-driven immune suppression within the tumor microenvironment.
Third, and most distinctively, Fc-enhanced ADCC activity introduces a cytotoxic effector mechanism capable of depleting PD-L1–expressing tumor and stromal cells. This additional layer may be particularly relevant in immune-cold tumors, where PD-L1–positive suppressive compartments limit effective immune activation.
Together, these coordinated mechanisms position IMM2510 as a multifunctional immuno-angiogenic platform, rather than a conventional combination compressed into one antibody.
Biomarker Evolution: A Dual-Axis Framework
If clinical development confirms activity, the biomarker strategy for IMM2510 will likely extend beyond PD-L1 alone toward a multidimensional model of response.
The immune axis would incorporate PD-L1 expression alongside interferon-γ–related inflammatory signatures, immune-inflamed transcriptional profiles, and spatial immune-cell infiltration patterns.
The angiogenic axis would evaluate VEGF signaling, hypoxia-associated biology, and broader angiogenesis-related transcriptional programs.
A dynamic monitoring axis could emerge through circulating tumor DNA kinetics or early molecular clearance as real-time indicators of therapeutic benefit.
This conceptual framework reflects the broader transformation in immuno-oncology—from reliance on single predictive markers toward integrated, context-aware biomarker ecosystems, consistent with contemporary bibliometric and translational trends in the field.
Key clinical question this class must answer
Does the added Fc-mediated killing improve outcomes without increasing immune-related toxicity or affecting activated immune cells? Preclinical efficacy supports the concept, but clinical translation will be the real test.
Key Takeaway Messages
- IMM2510 is a PD-L1 × VEGF bispecific designed to combine checkpoint blockade + anti-angiogenesis in one molecule.
- Its most differentiated feature is an Fc engineered for enhanced ADCC, aiming to eliminate PD-L1+ tumor/stromal cells—not just block signaling.
- A notable mechanistic signal is VEGF-enhanced cooperative PD-L1 binding, potentially increasing activity in VEGF-rich TMEs.
- Preclinical models (including NSCLC HCC827) show dose-dependent antitumor activity and superiority vs parental agents and combinations.
- If successful clinically, this class will likely require multi-axis biomarkers (immune + angiogenic + dynamic monitoring) rather than PD-L1 alone.
Conclusion
IMM2510 represents a mechanistically ambitious next-generation entry into the PD-(L)1×VEGF space. By integrating checkpoint blockade, VEGF ligand trapping, and Fc-enhanced effector function, it attempts to address resistance at multiple levels of the tumor immune ecosystem. The preclinical package is strong; the key next step is validating whether Fc-enabled cytotoxicity translates into meaningful clinical benefit with an acceptable safety profile—and which biomarker-defined subgroups stand to gain the most.
Read Full Article Here


