lidERA trial was presented at the 2025 San Antonio Breast Cancer Symposium (SABCS) by Aditya L. Bardia, MD (University of California, Los Angeles), reporting late-breaking results evaluating the next-generation oral selective estrogen receptor degrader (SERD) giredestrant versus standard-of-care endocrine therapy as adjuvant treatment for patients with estrogen receptor–positive (ER+), HER2-negative early breast cancer.
The study met its primary endpoint, demonstrating a statistically significant and clinically meaningful improvement in invasive disease-free survival (IDFS) with upfront giredestrant compared with standard endocrine therapy.
Background: The Need for Improved Adjuvant Endocrine Therapy
ER-positive, HER2-negative disease accounts for more than 70% of early breast cancers. Endocrine therapy remains the cornerstone of adjuvant treatment, with tamoxifen and aromatase inhibitors (AIs) forming the backbone of care. The last major advances in adjuvant endocrine therapy occurred in the early 2000s, when AIs demonstrated superiority over tamoxifen in trials such as ATAC and BIG 1-98, with disease-free survival hazard ratios of approximately 0.8.
More recently, CDK4/6 inhibitors combined with endocrine therapy have improved outcomes in selected high-risk patients, but at the cost of added toxicity. In routine practice, many patients discontinue endocrine therapy early due to tolerability issues, increasing the risk of recurrence. These limitations underscore the need for more effective and better-tolerated adjuvant endocrine options.
Giredestrant: Mechanistic Rationale
Giredestrant (GDC-9545) is a potent next-generation oral SERD and full estrogen receptor antagonist. The molecule is designed to bind the estrogen receptor and induce a conformational change that results in both full ER antagonism and ER degradation, leading to deep and sustained inhibition of ER signaling. Importantly, giredestrant inhibits both ligand-dependent and ligand-independent ER signaling, distinguishing it from aromatase inhibitors, which primarily block ligand-dependent pathways.
In preclinical cellular viability assays, giredestrant demonstrated greater potency than other SERDs across multiple ER-positive cell lines. In early breast cancer, giredestrant showed superior antiproliferative activity in the neoadjuvant setting in the coopERA Breast Cancer trial compared with anastrozole, and in the EMPR ESS trial compared with tamoxifen.
These findings provided the rationale for evaluating giredestrant as adjuvant therapy in the Phase III lidERA study.
Study Design
The lidERA Breast Cancer trial is a global, randomized, open-label, multicenter Phase III study. Patients with ER-positive, HER2-negative early breast cancer (AJCC stage I–III) were randomized 1:1 to receive either:
- Giredestrant 30 mg orally once daily, or
- Standard-of-care endocrine therapy, including tamoxifen or an aromatase inhibitor (anastrozole, letrozole, or exemestane).
Treatment duration was at least five years, with long-term follow-up planned. Premenopausal patients were required to receive ovarian function suppression if treated with giredestrant or an aromatase inhibitor; ovarian suppression was optional with tamoxifen, though most patients receiving tamoxifen also received ovarian suppression.
The primary endpoint was invasive disease-free survival (IDFS). Secondary endpoints included disease-free survival, distant recurrence-free interval (DRFI), overall survival (OS), and safety. The prespecified interim efficacy analysis of IDFS was conducted after 336 IDFS events were observed, corresponding to approximately 77% of events planned for the final analysis. The clinical cutoff date was August 8, 2025.
A total of 4,170 patients were randomized: 2,084 to giredestrant and 2,086 to standard-of-care endocrine therapy. In the control arm, 84% of patients received an aromatase inhibitor and 16% received tamoxifen.
Baseline demographics and disease characteristics were well balanced between the two arms. The median age was 54 years. Approximately 40% of patients were premenopausal. About half of the population had stage II disease, around 40% had stage III disease, and approximately 10% had stage I disease. The majority of patients had received prior chemotherapy before enrollment.
Median follow-up at the time of analysis was approximately 32.3 months.
Results of lidERA trial
The trial met its primary endpoint. Giredestrant significantly reduced the risk of invasive disease recurrence or death compared with standard-of-care endocrine therapy.
- IDFS hazard ratio: 0.70 (95% CI, 0.57–0.87; p = 0.0014)
- Risk reduction: 30%
- 3-year IDFS rates: 92.4% with giredestrant vs 89.6% with standard endocrine therapy
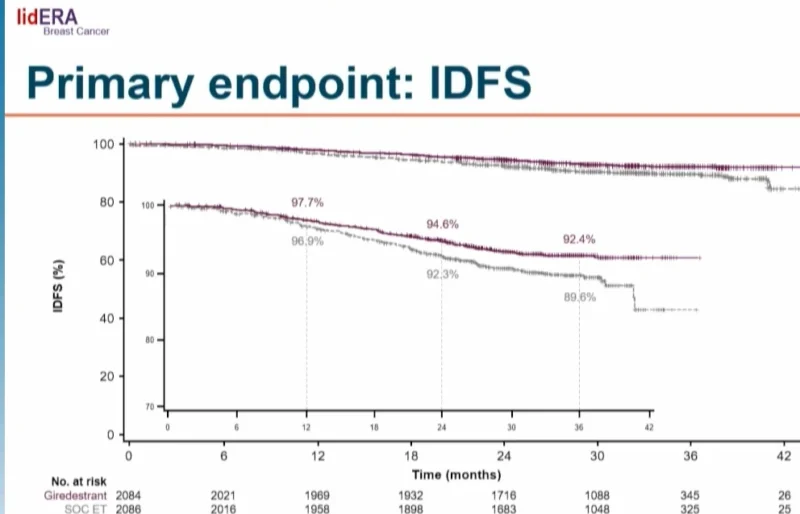
Kaplan–Meier curves separated early and remained separated over time.
When analyzed by type of standard endocrine therapy, giredestrant was superior to both aromatase inhibitors (HR 0.73) and tamoxifen (HR 0.53).
Subgroup Analyses
IDFS benefit with giredestrant was consistent across key prespecified subgroups, including age, region, menopausal status, risk category, and prior chemotherapy use.
By disease stage, the hazard ratio was 0.58 for stage II disease and 0.74 for stage III disease. In stage I disease, the confidence interval crossed 1, reflecting the small number of events in this lower-risk population.
Secondary Endpoints
Giredestrant significantly improved its secondary endpoint-distant recurrence-free interval compared with standard-of-care endocrine therapy.
- DRFI hazard ratio: 0.69 (95% CI, 0.54–0.89)
- Risk reduction: 31% in the development of distant metastatic disease
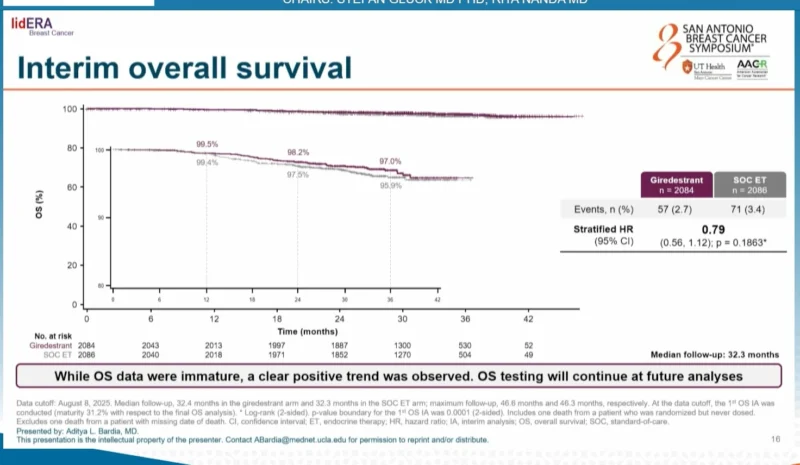
At the time of this interim analysis, overall survival data were immature. A positive trend favoring giredestrant was observed:
- OS hazard ratio: 0.79 (95% CI, 0.56–1.12; p = 0.1863)
- Further follow-up is ongoing to assess overall survival outcomes.
Safety
Safety analyses were conducted in the safety-evaluable population. Overall, adverse events, including grade 3–4 events and serious adverse events, were comparable between treatment arms.
Adverse events leading to treatment discontinuation occurred less frequently with giredestrant than with standard-of-care endocrine therapy (5.3% vs 8.2%). Total deaths were fewer in the giredestrant arm (6 vs 16).
Arthralgia was the most common adverse event in both arms. While the overall incidence of arthralgia was similar, arthralgia leading to treatment discontinuation was lower with giredestrant (1.6%) compared with standard endocrine therapy (3.7%).
Bradycardia was observed more frequently with giredestrant, but events were predominantly grade 1 and asymptomatic, with no grade 3 or 4 events reported. Grade 3–4 venous thromboembolic events were more frequent in the standard endocrine therapy arm, consistent with tamoxifen use.
Conclusions
With a median follow-up of 32.3 months, the lidERA Breast Cancer trial demonstrated that upfront giredestrant provides a statistically significant and clinically meaningful improvement in invasive disease-free survival compared with standard-of-care endocrine therapy in patients with ER-positive, HER2-negative stage I–III early breast cancer. The benefit was accompanied by improved distant recurrence-free interval, a favorable safety profile, and lower rates of treatment discontinuation.
Overall survival data are immature, but a positive trend favoring giredestrant was observed. These findings position giredestrant as a potential new standard of care in the adjuvant treatment of ER-positive, HER2-negative early breast cancer.
For more information click here.


