At the 2025 San Antonio Breast Cancer Symposium (SABCS), Dr. Erika Hamilton presented results from HER2CLIMB-05, a randomized, double-blind, phase III trial evaluating the addition of tucatinib to trastuzumab and pertuzumab (HP) as first-line maintenance therapy for patients with HER2-positive metastatic breast cancer (MBC) who had completed induction chemotherapy.
Clinical Background and Rationale
The current standard of care for first-line treatment of HER2-positive metastatic breast cancer consists of induction chemotherapy with a taxane in combination with dual HER2-targeted antibodies, trastuzumab and pertuzumab, followed by maintenance therapy with HP. Despite this approach, most patients eventually experience disease progression, and a proportion may not receive subsequent lines of therapy.
The original HER2CLIMB registrational trial demonstrated that adding tucatinib, a highly selective HER2 tyrosine kinase inhibitor, to capecitabine and trastuzumab significantly improved progression-free survival (PFS) and overall survival (OS) in heavily pretreated HER2-positive MBC, including patients with brain metastases. Based on this foundation, HER2CLIMB-05 was designed to test whether optimizing first-line maintenance therapy by targeting HER2 both extracellularly with monoclonal antibodies and intracellularly with tucatinib could further improve outcomes.
Study Design
HER2CLIMB-05 is an international, randomized, double-blind, placebo-controlled phase III trial (NCT05132582). Eligible patients had centrally confirmed HER2-positive metastatic breast cancer, ECOG performance status 0–1, and no evidence of disease progression after 4–8 cycles of taxane-based induction therapy with trastuzumab and pertuzumab (THP). Patients with asymptomatic brain metastases were allowed.
Participants were randomized 1:1 to receive:
- Tucatinib 300 mg orally twice daily plus HP, or
- Placebo plus HP,
administered every 21 days as maintenance therapy, with or without endocrine therapy when clinically indicated.
Randomization was stratified by disease status (de novo vs recurrent), hormone receptor status (positive vs negative), and presence or history of brain metastases.
The primary endpoint was investigator-assessed progression-free survival per RECIST v1.1.
Key secondary endpoints included overall survival, PFS by blinded independent central review (BICR), CNS-PFS, safety, health-related quality of life, and pharmacokinetics.
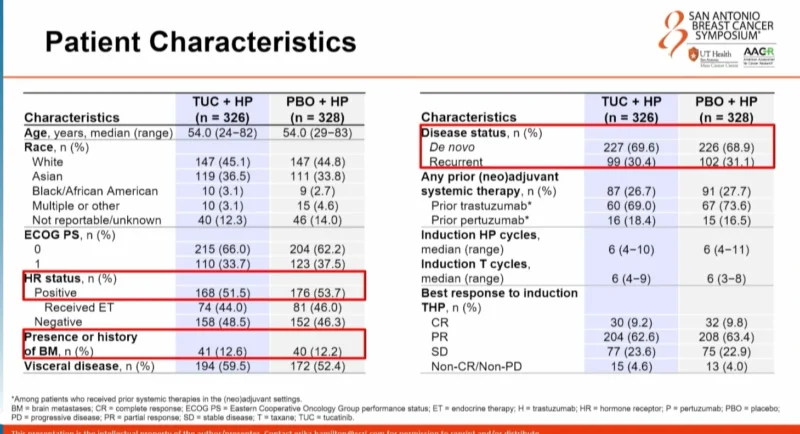
Patient Population
654 patients were randomized: 326 to tucatinib plus HP and 328 to placebo plus HP. At the September 5, 2025 data cutoff, 54.0% of patients in the tucatinib arm and 40.5% in the placebo arm remained on study treatment.
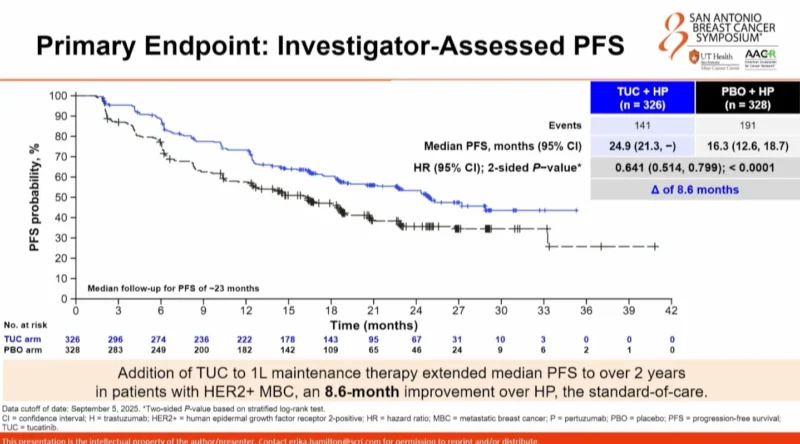
Primary Endpoint: Investigator-Assessed PFS
At a median follow-up of 23 months for PFS, the primary endpoint was met. Investigator-assessed median PFS was 24.9 months in the tucatinib plus HP arm compared with 16.3 months in the placebo plus HP arm, representing an absolute improvement of 8.6 months. This corresponded to a hazard ratio of 0.641 with a two-sided p-value of <0.0001.
The PFS benefit with tucatinib was observed consistently across all prespecified subgroups, including de novo versus recurrent disease, hormone receptor status, baseline brain metastases, and age (<65 vs ≥65years)
 .
.
In patients with hormone receptor–negative disease, median PFS improved from 12.6 months with placebo plus HP to 24.9 months with tucatinib plus HP, an absolute benefit of 12.3 months (HR 0.554; p=0.0002).
In those with hormone receptor–positive disease, median PFS improved from 18.1 months to 25.0 months, an absolute benefit of 6.9 months (HR 0.725; p=0.0389).
Secondary Endpoints
Blinded independent central review PFS was consistent with the primary analysis and demonstrated a median PFS of 28.9 months with tucatinib plus HP versus 16.0 months with placebo plus HP. The hazard ratio was 0.654, corresponding to a 12.9-month improvement.
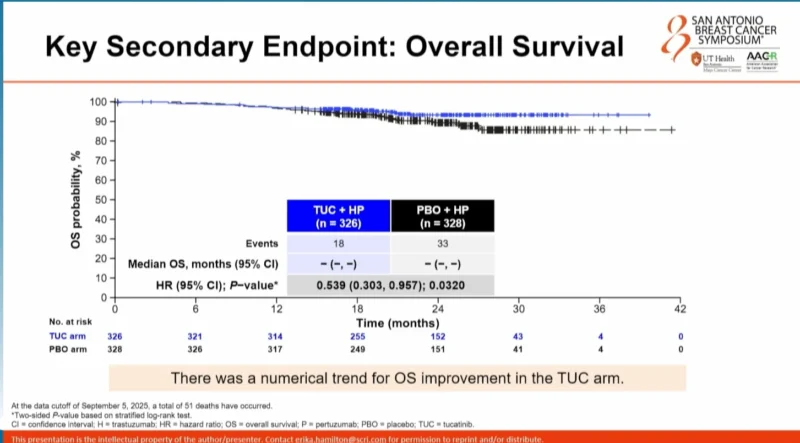
Median OS was not reached in either arm; however, a numerical trend favoring tucatinib plus HP was observed, with a hazard ratio of 0.539 (p=0.0320).
For CNS-PFS in the intention-to-treat population, median CNS-PFS was not reached in either arm. In an exploratory analysis of patients with baseline brain metastases, median CNS-PFS was 8.5 months with tucatinib plus HP compared with 4.3 months with placebo plus HP, corresponding to a 4.2-month difference, though this analysis was not statistically powered.
Safety and Tolerability
The addition of tucatinib resulted in a manageable safety profile consistent with prior experience. Grade ≥3 treatment-emergent adverse events occurred in 42.3% of patients receiving tucatinib plus HP and 24.4% of those receiving placebo plus HP. Discontinuation of tucatinib due to adverse events occurred in 13.5% of patients, most commonly due to hepatic events (7.7%).
Common treatment-emergent adverse events with tucatinib included diarrhea, nausea, ALT and AST elevations, arthralgia, and fatigue.
Conclusions
HER2CLIMB-05 demonstrated that the addition of tucatinib to first-line maintenance therapy with trastuzumab and pertuzumab resulted in a statistically significant and clinically meaningful improvement in progression-free survival in patients with HER2-positive metastatic breast cancer. The benefit was observed across all prespecified subgroups, including hormone receptor–positive and –negative disease.
Preliminary overall survival data showed a favorable trend for tucatinib plus HP, though follow-up remains immature. The combination exhibited a manageable safety profile, with adverse events largely consistent with known tucatinib toxicity and generally manageable through dose modifications.
These findings position tucatinib plus HP as an enhanced first-line maintenance therapy option, offering the potential to prolong time to disease progression and extend time off cytotoxic chemotherapy for patients with HER2-positive metastatic breast cancer.
For more information click here.


