Stage III non–small cell lung cancer (NSCLC) represents one of the most heterogeneous and challenging settings in thoracic oncology. Historically, concurrent chemoradiotherapy (cCRT) followed by observation was the standard for unresectable disease, yet long-term outcomes remained poor for decades.
This paradigm shifted with the PACIFIC trial, which established durvalumab maintenance after cCRT as a new standard of care, significantly improving progression-free and overall survival. However, clinical trial populations may not fully reflect routine practice. To address this gap, the GOECP-SEOR group conducted a large population-based study to characterize real-world treatment patterns and outcomes for stage III NSCLC in Spain.
Study Design and Methods
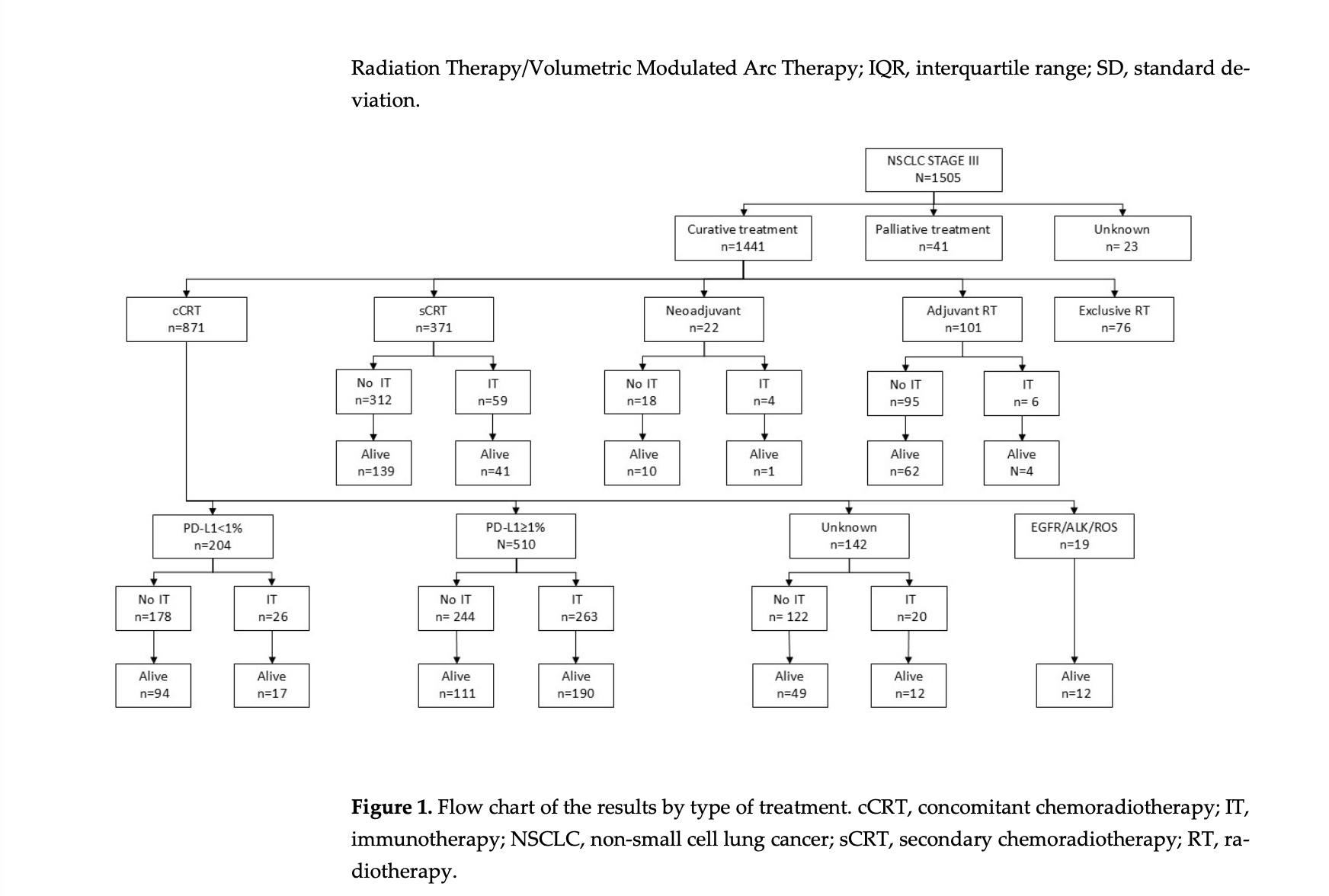
This was a retrospective, multicenter, observational study conducted across 35 Spanish radiation therapy departments. A total of 1,505 patients diagnosed with stage III NSCLC (AJCC 8th edition) and treated between 2018 and 2022 were included, capturing both pre- and post-PACIFIC eras. Patients received radiotherapy with curative, adjuvant, neoadjuvant, or palliative intent, with trimodal strategies allowed.
Data collected included demographics, tumor characteristics, treatment details, toxicity, progression, and survival outcomes. Overall survival (OS) and progression-free survival (PFS) were calculated from the end of radiotherapy. Multivariate Cox regression models were used to adjust for clinical and treatment-related factors.
Patient Characteristics
- Median age: 67 years
- Sex: 76% male
- Smoking history: 93% current or former smokers
- Performance status: ECOG 0 in >50% of patients
- Disease stage: Stage IIIA and IIIB comprised the majority of cases
- Histology: Squamous cell carcinoma was the most frequent subtype
PD-L1 status:
- Available in 56% of patients
- 73% of tested tumors had PD-L1 ≥1%
Treatment Patterns
The most common treatment approach was concurrent chemoradiotherapy, administered to 58% of patients, followed by sequential chemoradiotherapy in 24.7%. The mean radiotherapy dose was approximately 60 Gy, with IMRT/VMAT used in nearly 78% of cases and image-guided radiotherapy in over 87%, reflecting high technical standards.
Platinum-doublet chemotherapy was used in nearly three-quarters of patients. Surgery was performed in 10.4%, mainly in stage IIIA disease. Importantly, 26% of patients received immunotherapy, predominantly durvalumab according to the PACIFIC regimen, most often following cCRT.
Overall Survival
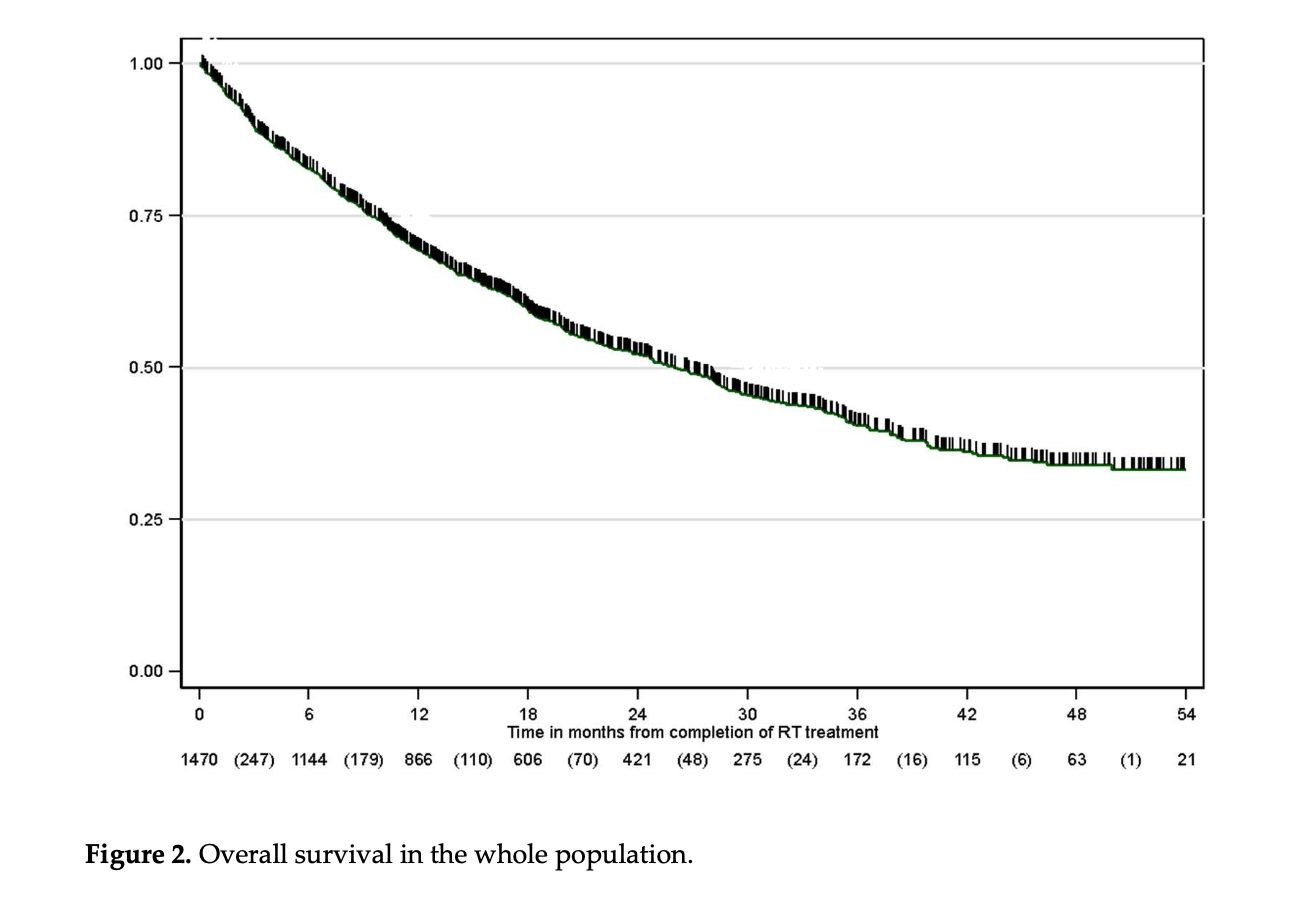
At a median follow-up extending to February 2023, 46.6% of patients had died, and the median overall survival for the entire cohort was 26 months. Among patients treated with radical cCRT, outcomes differed markedly by immunotherapy use. Median OS reached 43.9 months in patients receiving durvalumab compared with 19.4 months in those who did not (p < 0.001).
On multivariable analysis, durvalumab maintenance remained an independent predictor of improved OS (HR 0.42), even after adjustment for age, sex, stage, histology, and comorbidities. PD-L1 expression did not significantly stratify OS benefit, although numerically better outcomes were observed in patients with PD-L1 >50%.
Progression-Free Survival
Disease progression occurred in 64.7% of patients. Median PFS for the overall cohort was 11.2 months. Among cCRT-treated patients, median PFS was 20.8 months with durvalumab versus 8.4 months without immunotherapy (p < 0.001). Durvalumab remained strongly associated with improved PFS on multivariate analysis (HR 0.48).
Higher PD-L1 expression correlated with longer PFS, particularly in patients with PD-L1 ≥50%, though benefit was still observed across PD-L1 subgroups.
Safety
Treatment was generally well tolerated. Grade ≥3 toxicity occurred in 16.3% of patients overall. Severe pneumonitis (6–7%) and esophagitis (≈4%) were infrequent and comparable to rates reported in clinical trials. These findings support the feasibility of cCRT followed by immunotherapy in routine practice.
Discussion and Clinical Implications
This study represents one of the largest real-world datasets evaluating stage III NSCLC to date. Treatment practices in Spanish radiation oncology departments closely aligned with international guidelines, with a notably high use of cCRT and modern radiotherapy techniques. The real-world effectiveness of durvalumab maintenance mirrored, and in some cases exceeded, outcomes reported in the PACIFIC trial and other registry studies.
The findings reinforce durvalumab consolidation as a key determinant of survival in unresectable stage III NSCLC and support its use beyond strictly selected trial populations. Importantly, benefit was observed regardless of PD-L1 status, raising questions about current access restrictions in some healthcare systems.
You Can Watch More on OncoDaily Youtube TV


