Postoperative ctDNA (molecular residual disease, MRD) is emerging as a strong prognostic tool in colorectal cancer, but most rectal cancer data come from cohorts exposed to neoadjuvant therapy/TNT, which complicates interpretation. This prospective analysis asked a simpler question: in stage II–III rectal cancer treated with upfront surgery, can postoperative ctDNA (1) predict recurrence risk and (2) identify who actually benefits from adjuvant chemotherapy (ACT)?
Study Design and Methods
This analysis was embedded within the prospective Japanese GALAXY study (CIRCULATE Japan platform) and examined a clinically clean setting: pathologic stage II–III rectal cancer treated with upfront, curative-intent (R0) surgery, with postoperative ctDNA testing available. The evaluable cohort included 250 patients.
ctDNA was assessed using Signatera, a personalized, tumor-informed 16-plex PCR–NGS assay built from each patient’s tumor/normal sequencing; plasma samples were classified as ctDNA-positive when ≥2 tumor-specific variantswere detected above the prespecified threshold. Blood collection was structured around clinically meaningful postoperative intervals, anchored by an MRD window (2–10 weeks post-surgery, prior to initiation of adjuvant chemotherapy), followed by serial surveillance with additional landmarked assessments at approximately 3 months and 6 months and continued monitoring in a defined surveillance period.
GALAXY Study
The primary endpoint was disease-free survival (DFS), measured from prespecified landmark time points to mitigate immortal time bias inherent to postoperative biomarker sampling. The central analytic goal was twofold: first, to determine whether postoperative ctDNA status robustly stratifies recurrence risk after upfront surgery; and second, to test whether ctDNA functions as a predictive biomarker for adjuvant chemotherapy benefit, distinguishing patients likely to benefit from ACT from those unlikely to derive measurable DFS improvement.
Results
Postoperative ctDNA emerged as a powerful and consistent determinant of recurrence risk and treatment benefit.
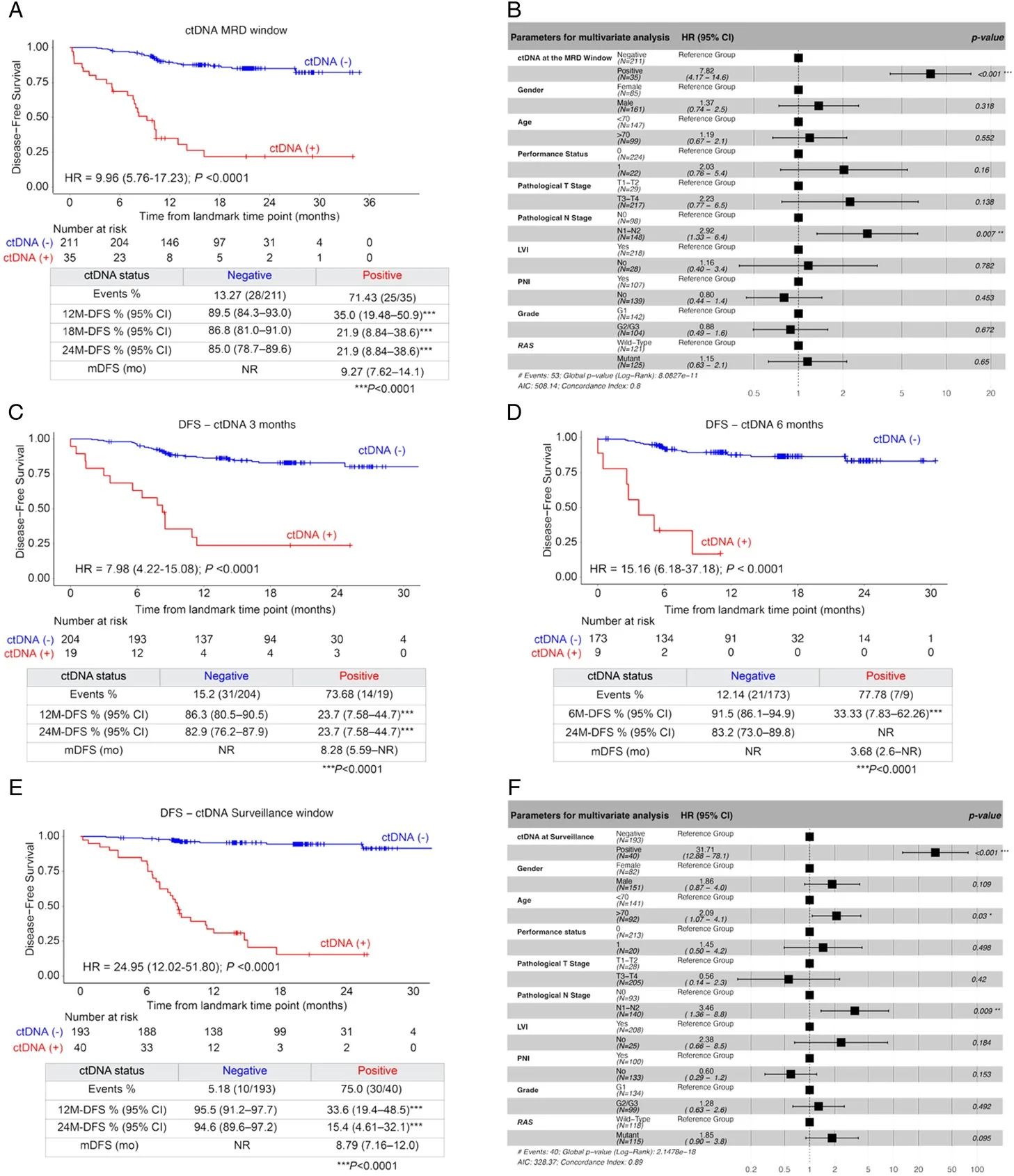
First, ctDNA status in the postoperative MRD window (2–10 weeks after surgery) sharply separated patients into distinct risk groups. Although only 14.2% of evaluable patients were ctDNA-positive at this time point, this subgroup experienced profoundly inferior disease-free survival compared with ctDNA-negative patients (HR 9.96, 95% CI 5.76–17.2; P<0.0001). The clinical impact was striking, with 12-month DFS of 35.0% in ctDNA-positive patients versus 89.5% in ctDNA-negative patients. In multivariable models, MRD ctDNA positivity was the strongest prognostic factor, outweighing most conventional clinicopathologic variables; only pathologic nodal stage retained independent prognostic significance alongside ctDNA.
Second, the risk-stratifying value of ctDNA persisted—and intensified—over time. ctDNA positivity at 3 months and 6 months post-surgery remained strongly associated with recurrence (HR 7.98 and HR 15.16, respectively; both P<0.0001). During the surveillance window, any ctDNA positivity conferred an almost 25-fold higher risk of recurrence compared with patients who remained serially ctDNA-negative (HR 24.95, P<0.0001). Notably, in surveillance multivariable analyses, ctDNA positivity became even more dominant, with a hazard ratio exceeding 30, underscoring its central role as a dynamic risk marker.
Third, ctDNA status appeared to be predictive of adjuvant chemotherapy benefit, not merely prognostic. Among patients who were ctDNA-negative in the MRD window, adjuvant chemotherapy did not confer a statistically significant DFS advantage (HR 0.59, P=0.211). In contrast, ctDNA-positive patients derived clear benefit from ACT, with a 72% reduction in recurrence risk (HR 0.28, P=0.031) and an improvement in median DFS (9.33 months with ACT vs 5.62 months with observation). This differential effect supports ctDNA as a tool for treatment selection, rather than simple risk stratification.
Fourth, ctDNA dynamics over time carried critical prognostic information. When examining patients with both MRD and 6-month ctDNA data (using landmark analyses to account for survival to 6 months), outcomes followed a clear hierarchy. Patients who remained persistently ctDNA-negative had the best prognosis. Those who converted from negative to positive faced a markedly increased recurrence risk (HR 8.22, P=0.0055), while patients who were persistently ctDNA-positive had an exceptionally high risk (HR 45.48, P<0.0001). These findings highlight the danger of molecular conversion and the value of serial monitoring.
Finally, the study revealed an important biologic and site-specific nuance: lung metastases were more likely to be ctDNA-negative early after surgery, consistent with known patterns of lower ctDNA shedding from pulmonary lesions. Although many of these patients eventually became ctDNA-positive with longitudinal testing, this observation emphasizes the need for cautious interpretation of early ctDNA-negative results in clinical contexts where lung-only relapse risk is high, and further supports the rationale for repeated, longitudinal ctDNA assessment.
Insights
- In upfront-surgery rectal cancer, postoperative MRD ctDNA is an exceptionally strong marker of recurrence risk—the hazard ratios here are not subtle.
- The data suggest ctDNA is not only prognostic but also predictive: ACT benefit appears concentrated in MRD-positive patients, while MRD-negative patients did not show a measurable DFS advantage.
- Serial ctDNA adds real value: patients can “declare themselves” later (molecular recurrence) before imaging, enabling earlier intervention—at least conceptually.
Conclusion
For stage II–III rectal cancer treated with upfront surgery, postoperative ctDNA is a robust MRD biomarker that strongly predicts recurrence and appears to identify who benefits from adjuvant chemotherapy. The work supports a future where ACT intensity and surveillance are personalized based on ctDNA status and dynamics, with the caveat that treatment paradigms differ across regions and randomized ctDNA-guided trials remain essential for practice-changing adoption.
You Can Read All Article here