The FORTITUDE-101 trial, presented by Prof. Sun Young Rha at the ESMO Congress 2025 during Presidential Symposium , evaluated bemarituzumab (BEMA), a first-in-class anti-FGFR2b monoclonal antibody, in combination with mFOLFOX6 chemotherapy for patients with FGFR2b-overexpressing, non-HER2-positive, unresectable, or metastatic gastric and gastroesophageal junction cancer (G/GEJC). BEMA simultaneously blocks oncogenic FGFR2b signaling and activates antibody-dependent cell-mediated cytotoxicity (ADCC), offering a novel therapeutic approach for this biomarker-defined subgroup with poor prognosis.
Background
FGFR2b overexpression is observed in a substantial proportion of gastric and gastroesophageal junction cancers (G/GEJC) and is recognized as a driver of tumor aggressiveness, higher metastatic potential, and poorer survival outcomes. Patients with this biomarker often experience rapid disease progression and limited response to conventional chemotherapy.
Currently, standard first-line treatment options—typically based on platinum and fluoropyrimidine-containing regimens—provide only modest benefit, leaving a significant unmet need for biomarker-directed targeted therapies in this population.
To address this gap, the phase 3 FORTITUDE-101 study (NCT05052801) was designed to evaluate whether the addition of bemarituzumab (BEMA), a first-in-class anti-FGFR2b monoclonal antibody, to mFOLFOX6 chemotherapy could offer a clinically meaningful survival advantage compared with placebo plus mFOLFOX6. The trial specifically focused on patients with tumors showing FGFR2b expression in ≥10% of tumor cells (2+/3+ intensity), aiming to establish a new standard for FGFR2b-targeted therapy in advanced G/GEJC.
Methods
A total of 547 patients with FGFR2b-overexpressing, non-HER2-positive, unresectable or metastatic G/GEJC were enrolled and randomized 1:1 to receive either:
- BEMA 15 mg/kg every 2 weeks (Q2W) with an additional 7.5 mg/kg on cycle 1 day 8, plus mFOLFOX6, or
- Matched placebo plus mFOLFOX6.
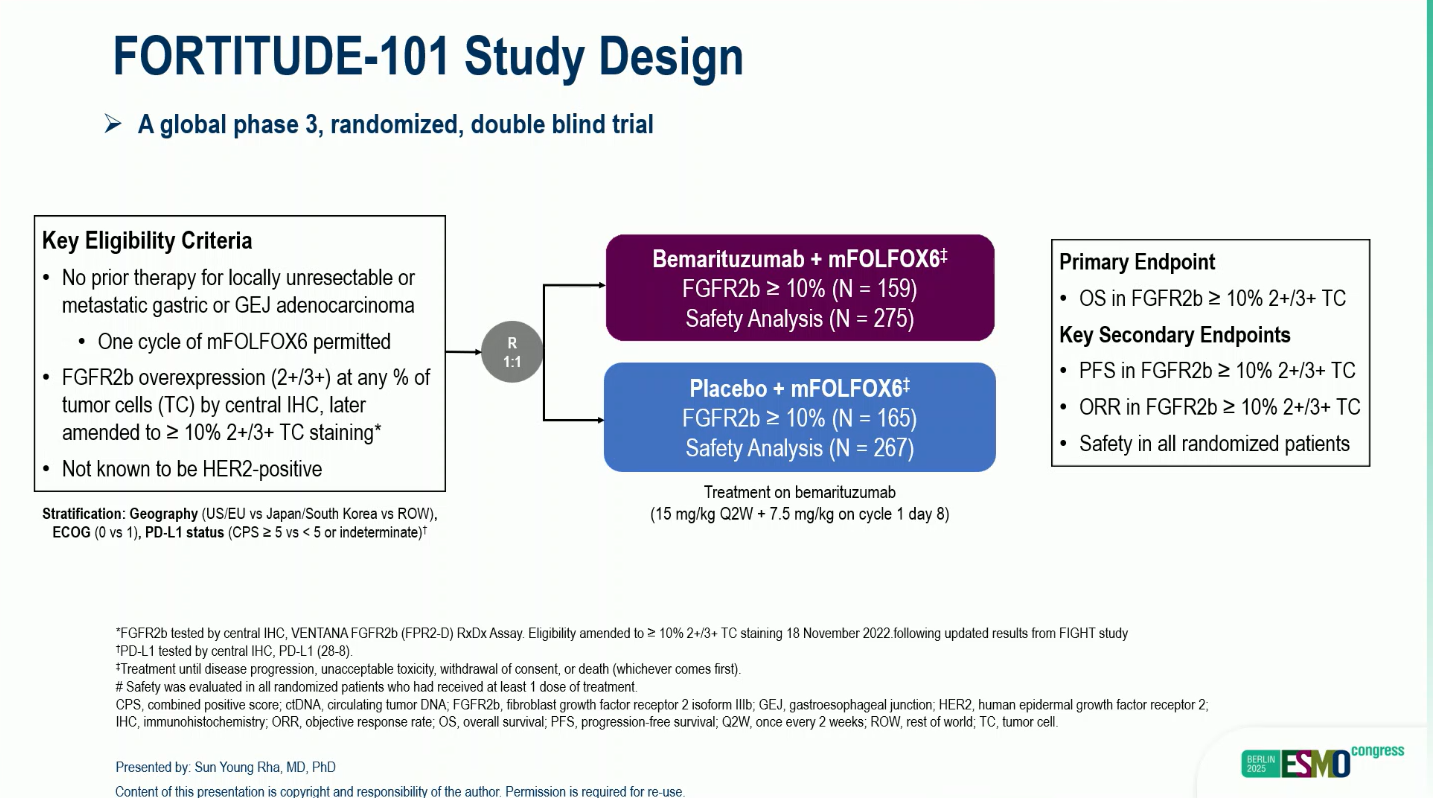
The primary endpoint was overall survival (OS) in patients with FGFR2b ≥10% 2+/3+ tumor cell staining, with progression-free survival (PFS) and objective response rate (ORR) as key secondary endpoints.
An interim analysis (primary analysis, PA) was performed at a data cutoff of December 9, 2024, after crossing pre-specified efficacy boundaries. A follow-up descriptive analysis (FA) was conducted at a later cutoff (June 20, 2025).
Results
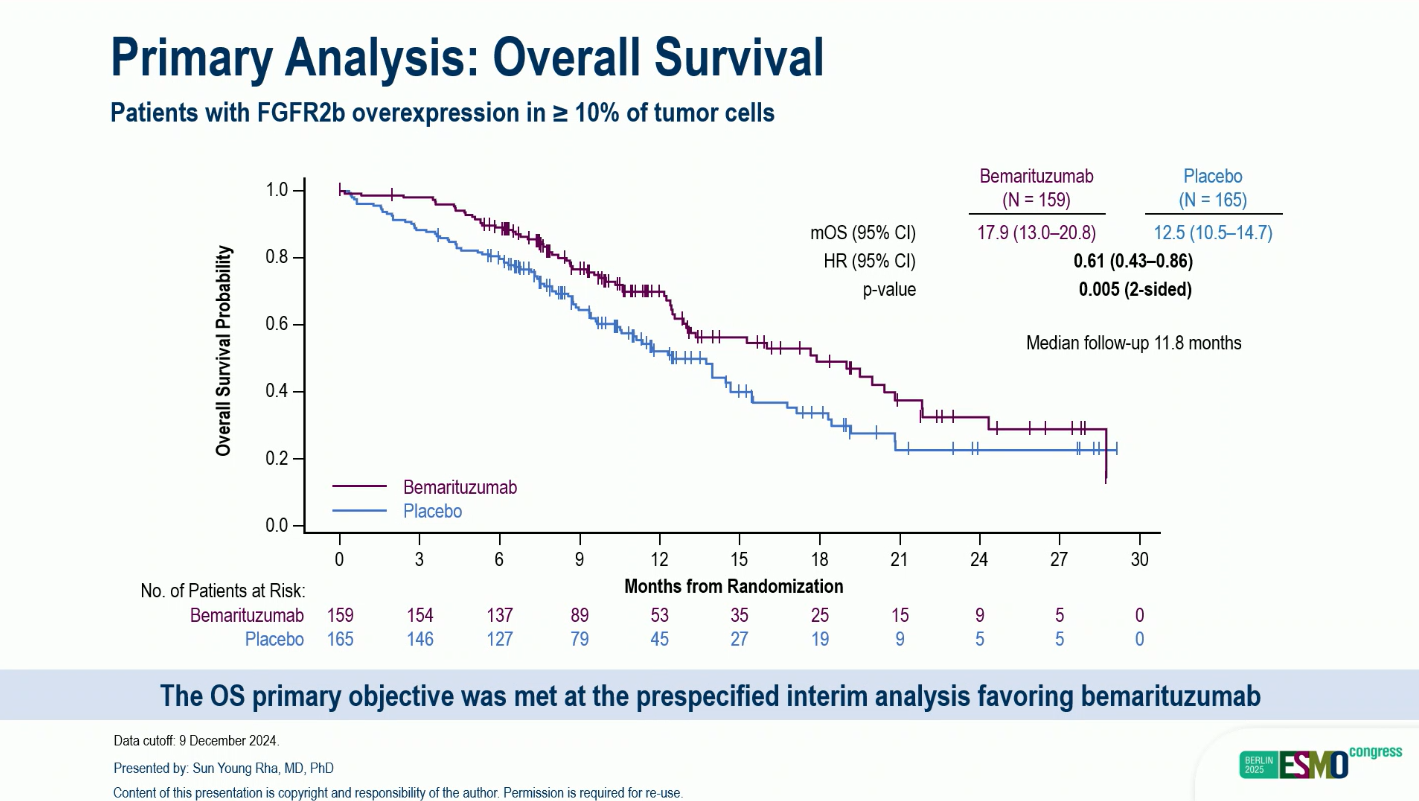
At the primary analysis (median follow-up 11.8 months):
- Median OS: 17.9 months (BEMA) vs 12.5 months (placebo)
- Hazard ratio (HR): 0.61 (95% CI 0.43–0.86); P = 0.005
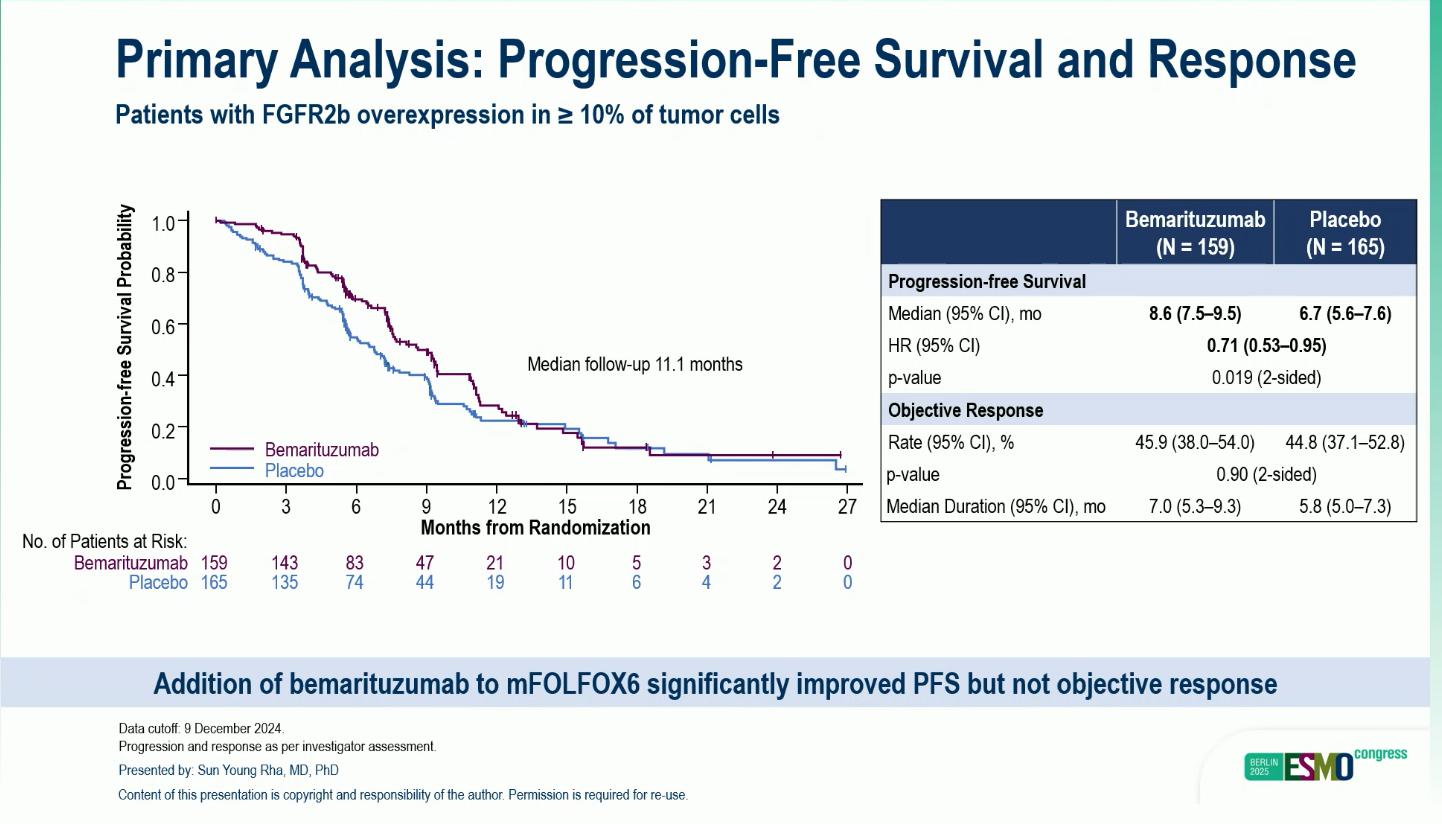
- Median PFS: 8.6 months vs 6.7 months; HR 0.71 (95% CI 0.53–0.95); P = 0.019
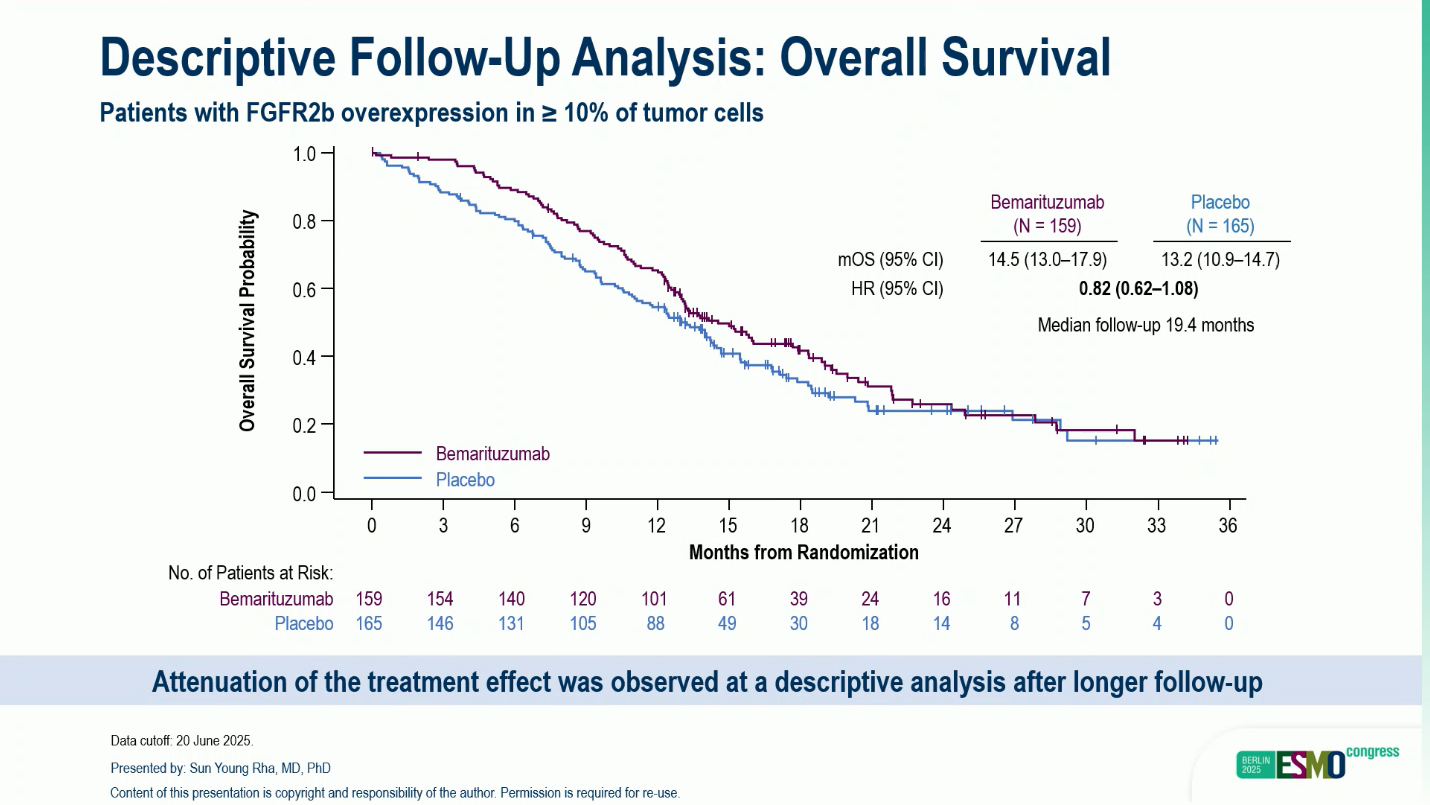
At the follow-up analysis (median follow-up 19.4 months), median OS was 14.5 months vs 13.2 months (HR 0.82, 95% CI 0.62–1.08), indicating some attenuation of treatment effect over time but sustained clinical benefit in early analyses.
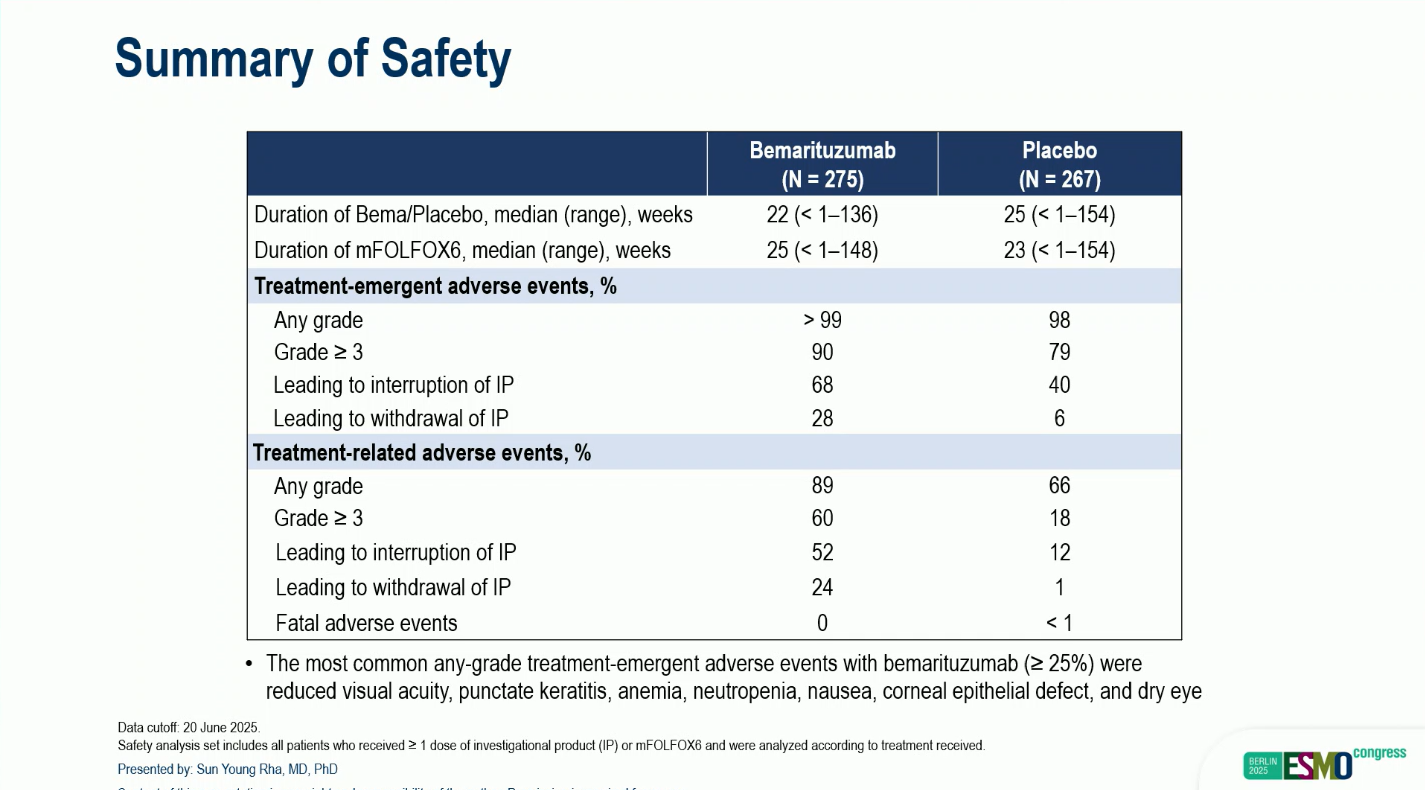
Safety:
- Any-grade treatment-emergent adverse events (TEAEs): >99%(BEMA) vs 98% (placebo)
- Grade ≥3 TEAEs: 90% vs 79%
- Treatment-related Grade ≥3 AEs: 60.% vs 18%, mainly due to corneal toxicities, consistent with FGFR inhibition.
Conclusions
The FORTITUDE-101 trial met its primary endpoint of overall survival (OS) and key secondary endpoint of progression-free survival (PFS) in patients with FGFR2b-overexpressing gastric and gastroesophageal junction cancer (≥10% of tumor cells), establishing FGFR2b as a validated therapeutic target in this population.
While the OS benefit observed at the primary analysis was statistically significant, it attenuated at a subsequent descriptive follow-up, likely reflecting post-progression treatments and longer observation. Nonetheless, early and durable gains in both OS and PFS support the clinical activity of bemarituzumab (BEMA) in combination with mFOLFOX6.
The safety profile of bemarituzumab was primarily driven by corneal adverse events, including transient decreases in visual acuity. Importantly, these ocular effects were mostly reversible with appropriate management, underscoring the drug’s manageable tolerability in the context of targeted FGFR inhibition.
Results from FORTITUDE-101, together with ongoing evaluation in the FORTITUDE-102 trial (NCT05111626), will further define the benefit–risk profile of bemarituzumab in FGFR2b-overexpressing gastric and GEJ cancer, potentially shaping future first-line standards of care for this biomarker-selected subgroup.
You can read the full abstract here.


