Immune checkpoint inhibitors (ICIs) have significantly improved outcomes in non-small cell lung cancer (NSCLC) and melanoma; however, primary resistance remains common, affecting approximately half of treated patients. Increasing evidence implicates the gut microbiome as a critical modulator of immunotherapy response. Prior phase I studies suggested that fecal microbiota transplantation (FMT) could overcome resistance to PD-1 blockade, but its efficacy and safety in first-line NSCLC and in combination with dual PD-1/CTLA-4 blockade had not been prospectively evaluated.
Study Design and Methods
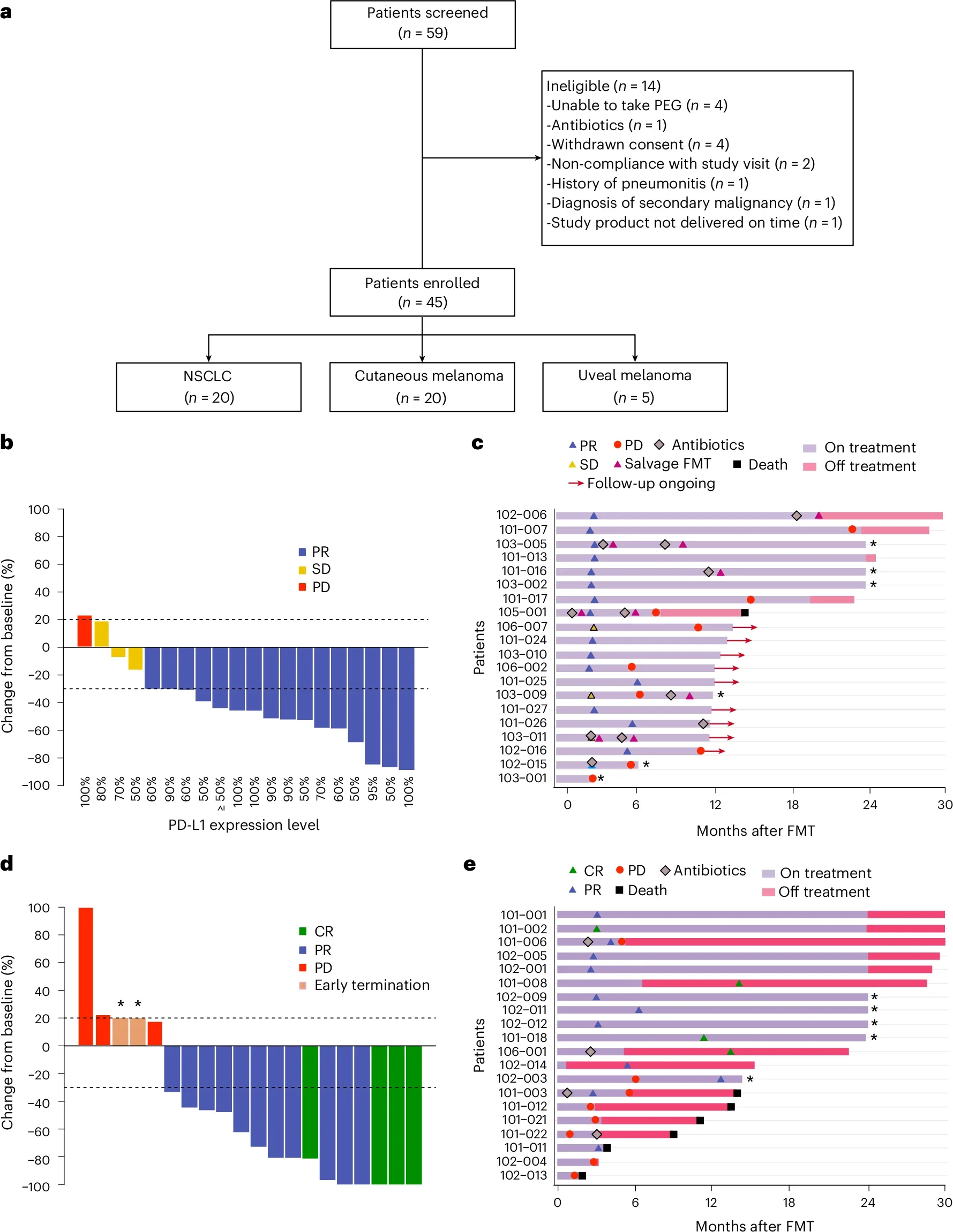
FMT-LUMINate was a multicenter, open-label, phase II trial evaluating healthy-donor FMT combined with immune checkpoint inhibition in the first-line setting. Patients received a single oral FMT prior to immunotherapy initiation.
- NSCLC cohort (n = 20):
PD-L1 TPS ≥50%, no actionable oncogenic alterations
Treatment: FMT → pembrolizumab monotherapy - Melanoma cohort (n = 20):
Cutaneous melanoma, regardless of BRAF status
Treatment: FMT → nivolumab + ipilimumab
Primary endpoint: Objective response rate (ORR) in NSCLC
Secondary endpoints: ORR in melanoma, safety, microbiome dynamics, and donor–host similarity
FMT-LUMINate Trial
Key Results
NSCLC
- ORR: 80% (16/20), exceeding the prespecified efficacy threshold
- Disease control rate: 95%
- 1-year progression-free survival: 65%
- 1-year overall survival: 100%
Melanoma
- ORR: 75% (11 partial responses, 4 complete responses)
- 1-year progression-free survival: 58%
- 1-year overall survival: 79%
These response rates exceeded historical benchmarks for pembrolizumab monotherapy in PD-L1–high NSCLC and for nivolumab plus ipilimumab in melanoma.
FMT-LUMINate Trial
Safety
NSCLC cohort
- No grade ≥3 adverse events
- Toxicities consistent with known pembrolizumab safety profile
Melanoma cohort
- Grade ≥3 adverse events in 65% of patients
- Diarrhea/colitis most common severe toxicity
- Myocarditis observed in 15%, with earlier onset than historically reported
- Severe toxicities clustered in recipients of Prevotella-rich donor FMT
The combination was deemed acceptable by the independent safety monitoring committee, but myocarditis was designated an adverse event of special interest for future trials.
Mechanistic Insights
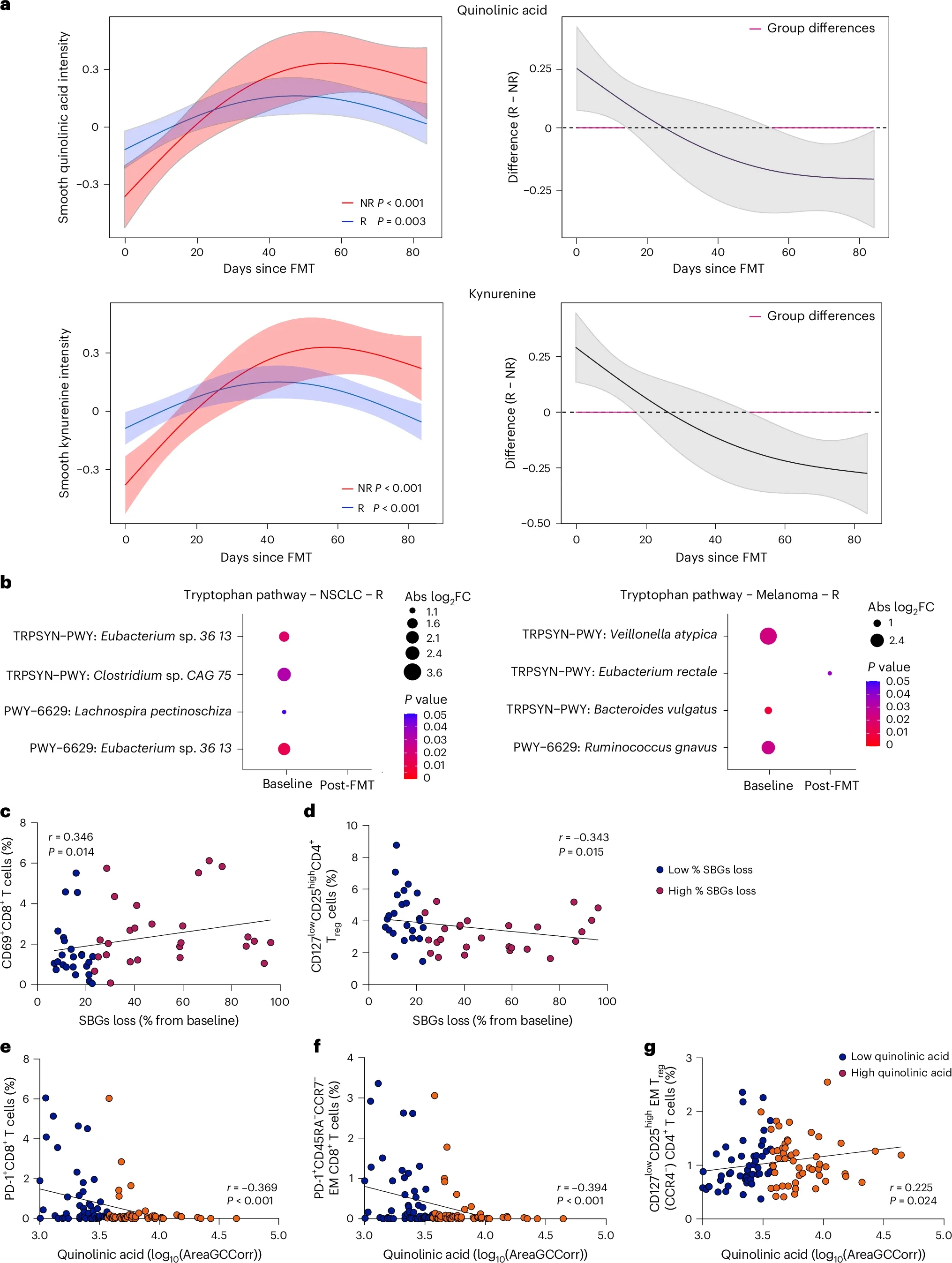
Mechanistic analyses demonstrated that clinical response to fecal microbiota transplantation was not driven by acquisition of donor-derived microbial strains or by increased donor–recipient microbiome similarity. Measures of global microbiome similarity and strain-level engraftment were comparable between responders and non-responders, indicating that donor microbial engraftment alone did not account for treatment efficacy. Instead, responders exhibited a markedly greater loss of bacterial species present at baseline compared with non-responders. The taxa most consistently depleted in responding patients included species from the Enterocloster genus, Clostridium innocuum, Dialister, and Streptococcus. This pattern of baseline bacterial loss was reproducible across three independent clinical trials evaluating fecal microbiota transplantation in combination with immune checkpoint inhibition, supporting its generalizability.
The functional relevance of this microbial depletion was confirmed in preclinical models. Reintroduction of bacterial species that had been eliminated following fecal microbiota transplantation into tumor-bearing mice abrogated the antitumor activity of PD-1 blockade, both alone and in combination with CTLA-4 inhibition. These findings indicate that removal of deleterious bacterial taxa is required for the full therapeutic benefit of fecal microbiota transplantation when combined with immune checkpoint inhibitors.
At a systemic level, loss of these baseline taxa was associated with distinct immunometabolic changes. Responders demonstrated reduced circulating levels of kynurenine and quinolinic acid, key metabolites of the tryptophan pathway that have been linked to immunosuppression and resistance to immunotherapy. This metabolic shift was accompanied by an increase in effector CD8⁺ T-cell populations and a concomitant reduction in regulatory T cells, consistent with the development of a more immunostimulatory systemic immune environment following fecal microbiota transplantation.
Clinical Implications
FMT-LUMINate provides the first prospective phase II evidence that healthy-donor FMT can enhance immunotherapy efficacy in:
- PD-L1–high NSCLC treated with anti-PD-1 monotherapy
- Melanoma treated with dual PD-1/CTLA-4 blockade
Importantly, therapeutic benefit appears to depend on elimination of immunosuppressive gut bacteria, rather than simple microbial engraftment. Donor microbiome composition, particularly Prevotella enrichment, may influence toxicity in the context of CTLA-4 blockade.
Conclusion
The FMT-LUMINate trial establishes fecal microbiota transplantation as a biologically active immunomodulatory strategy capable of enhancing checkpoint inhibitor efficacy across tumor types and treatment backbones. These findings support the development of next-generation microbiome-based therapies designed to deplete deleterious pathobionts, refine donor selection, and personalize immunotherapy combinations.
You Can Read All article Here.