Presented at ASCO 2025 by Dr. Anne-Claire Hardy-Bessard, the FIRST/ENGOT-OV44 Phase III trial (NCT03602859) evaluated whether adding dostarlimab, a PD-1 immune checkpoint inhibitor, to standard first-line platinum-based chemotherapy (PBCT) followed by niraparib maintenance therapy (MT) ± bevacizumab could improve outcomes in patients with newly diagnosed advanced ovarian cancer (aOC).
FIRST/ENGOT-OV44 Trial Design and Patient Enrollment
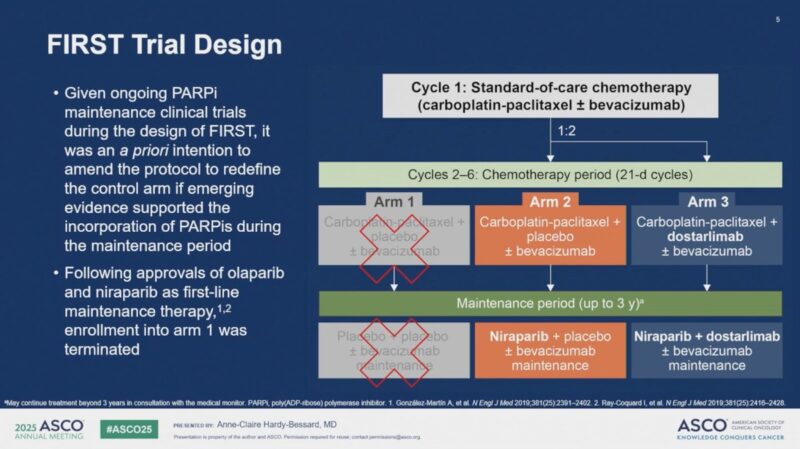
FIRST/ENGOT-OV44 was a randomized, double-blind, phase III study evaluating whether the PD-1 inhibitor dostarlimab could enhance outcomes when added to first-line PBCT and niraparib maintenance in patients with stage III–IV high-grade nonmucinous epithelial ovarian cancer.
After a one-cycle run-in of PBCT ± bevacizumab (bev), patients were randomized in a 1:1:2 ratio to:
- Arm 1: PBCT + placebo (PBO), followed by PBO maintenance
- Arm 2: PBCT + PBO, followed by niraparib + PBO maintenance
- Arm 3: PBCT + dostarlimab, followed by niraparib + dostarlimab maintenance
Stratification was based on BRCA/HRR status, intent to use bevacizumab, and postoperative residual disease status. Following the regulatory approval of PARP inhibitors in the first-line maintenance setting, enrollment to Arm 1 was discontinued. Patients were subsequently randomized 1:2 to Arms 2 and 3 only.
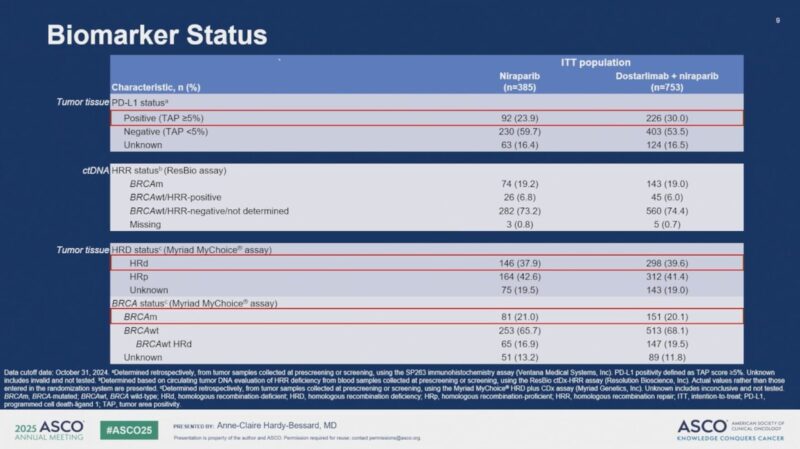
In total, 1,138 patients were randomized between November 2018 and January 2021, with 385 assigned to Arm 2 and 753 to Arm 3. The study population included a broad representation of disease characteristics: 37.3% had stage IV disease at diagnosis, 39% had homologous recombination-deficient (HRd) tumors, and 33.4% were PD-L1 positive among those with available biomarker data. The median follow-up at the data cutoff (October 31, 2024) was 45.9 months.
Key Results Presented at ASCO 2025
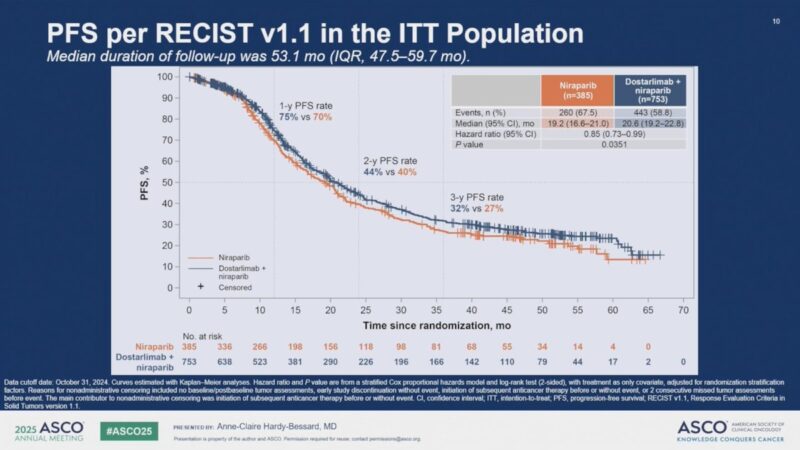
The primary endpoint of the study—investigator-assessed progression-free survival (PFS) per RECIST v1.1—was met, with a statistically significant improvement seen in patients receiving dostarlimab. Median PFS was 20.63 months in Arm 3 (dostarlimab + chemotherapy + niraparib maintenance) compared to 19.19 months in Arm 2 (chemotherapy + niraparib maintenance alone), with a hazard ratio (HR) of 0.85 (95% CI: 0.73–0.99; P = 0.0351).
Subgroup analyses, although not powered for statistical significance, showed favorable trends with dostarlimab across several patient populations. In patients with PD-L1–positive tumors, the HR was 0.84 (95% CI: 0.61–1.17). Those with homologous recombination-deficient (HRd) tumors had an HR of 0.95 (95% CI: 0.72–1.24). The benefit of dostarlimab was also observed regardless of bevacizumab use: patients treated with bevacizumab had an HR of 0.84 (95% CI: 0.68–1.03), while those not receiving bevacizumab had an HR of 0.86 (95% CI: 0.69–1.08).
Despite the improvement in PFS, overall survival (OS)—a key secondary endpoint—was not significantly different between the two arms. Median OS was 44.39 months in the dostarlimab group versus 45.37 months in the control group, yielding an HR of 1.01 (95% CI: 0.86–1.19; P = 0.9060).
Safety
The safety analysis included 1,321 patients across all three treatment arms. This population included 34 patients originally randomized to arm 1 who, after unblinding, received treatment aligned with arm 2. Additionally, 10 randomized patients did not receive any study treatment and were excluded from the safety evaluation.
The median duration of treatment exposure was 11.3 months in arm 1, and 15.2 months in both arms 2 and 3. Importantly, the safety findings were consistent with the known toxicity profiles of each agent used in the study—dostarlimab, niraparib, and platinum-based chemotherapy—with no new safety signals identified.
Overall, the combination of dostarlimab and niraparib, whether used with or without bevacizumab, was generally well tolerated, supporting its feasibility as part of a first-line treatment strategy for advanced ovarian cancer.
Read Full Abstract on ASCO Official Website
What People Are Saying About FIRST/ENGOT-OV44?
Dr. Taro Yamanaka shared his thoughts on the results of the FIRST/ENGOT-OV44 trial on his X account, summarizing the key outcomes and raising a critical question about patient selection:
“Ph3 FIRST/ENGOT-OV44 in 1L advanced #OvarianCancer
Statistically significant PFS benefit with dostarlimab (20.6 vs 19.2 mo; HR 0.85, P=0.0351)
No OS gain (HR 1.01)
Absolute benefit of ICI combination seems modest…
Who truly benefits from ICI?”
Key Takeaway
The addition of dostarlimab to first-line chemotherapy and niraparib maintenance significantly prolonged progression-free survival in patients with newly diagnosed advanced ovarian cancer. Although no OS benefit was seen, this regimen represents a potential new option in the frontline setting, warranting further exploration in biomarker-defined subgroups.
More posts featuring ASCO25.


