The Evolution trial (WJOG11819L) was designed to test whether radiotherapy could be safely omitted in selected patients with unresectable, locally advanced non-small-cell lung cancer (NSCLC) exhibiting high PD-L1 expression (TPS ≥50%). This patient population represents an immunologically distinct subgroup known to derive substantial benefit from PD-1 blockade, as demonstrated in prior pivotal studies such as KEYNOTE-024, -042, -189, and -407, which established pembrolizumab-based regimens as the foundation of systemic therapy in advanced NSCLC.
In this phase 2, multicentre, single-arm study, investigators sought to determine whether pembrolizumab combined with platinum-based chemotherapy, administered without radiotherapy, could achieve durable disease control and survival outcomes comparable to the standard chemoradiotherapy followed by durvalumab consolidation used in the PACIFIC trial. The rationale was to explore a radiotherapy-free, chemoimmunotherapy approach that could reduce treatment-related toxicities such as pneumonitis and esophageal injury—complications that frequently limit the feasibility of curative-intent chemoradiation and preclude up to one-third of patients from receiving consolidation immunotherapy.
Study Design and Methods
This multicentre, single-arm, phase 2 trial was conducted across nine Japanese centers under the West Japan Oncology Group (WJOG).
Eligible patients were adults (≥20 years) with unresectable, locally advanced stage IIIA–IIIC NSCLC, PD-L1 TPS ≥50%, ECOG performance status 0–1, and no prior systemic therapy. All had an indication for definitive chemoradiotherapy but received a radiotherapy-free regimen instead.
Treatment protocol:
Induction therapy (4 cycles): Pembrolizumab 200 mg every 3 weeks + platinum-based doublet chemotherapy
- Non-squamous: Cisplatin or carboplatin + pemetrexed
- Squamous: Carboplatin + nab-paclitaxel
Maintenance therapy: Pembrolizumab (± pemetrexed) every 3 weeks for up to 2 years.
- Primary endpoint: 2-year progression-free survival (PFS)
- Secondary endpoints: Objective response rate (ORR), overall survival (OS), duration of response (DOR), and safety.
- Statistical threshold: A 2-year PFS rate ≥20% was considered clinically meaningful; ≥45% was expected based on PACIFIC trial benchmarks.
Results
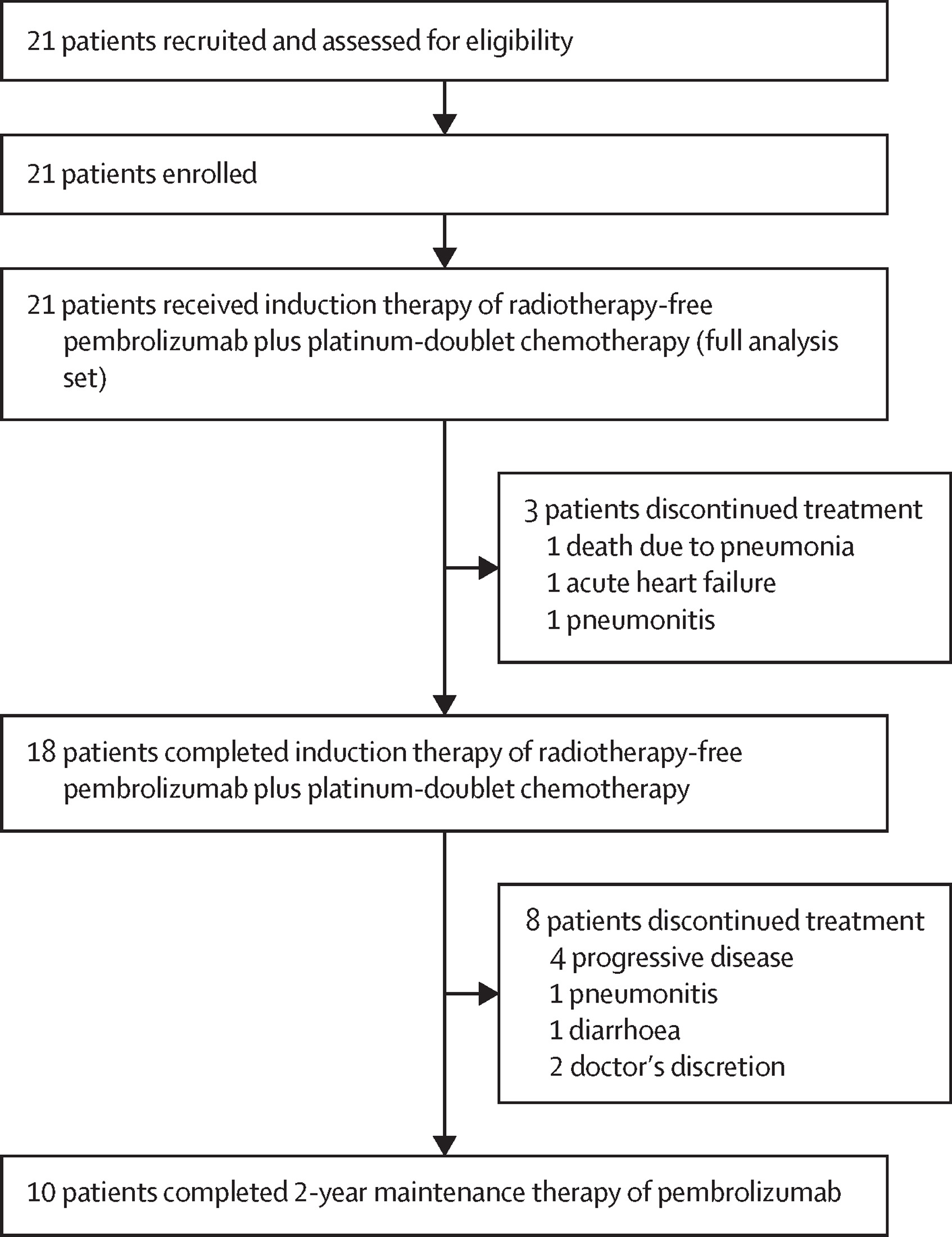
Between May 18, 2020, and February 22, 2022, a total of 21 patients were enrolled in the Evolution trial. The median age was 73 years, and 76% were male. All patients received pembrolizumab combined with carboplatin-based chemotherapy, followed by maintenance pembrolizumab. Nearly half of the patients (48%) were able to complete the full 2-year course of maintenance therapy. The median follow-up period was 32.5 months, allowing for mature outcome assessment.
Efficacy Outcomes
The primary endpoint was met, demonstrating strong and durable disease control:
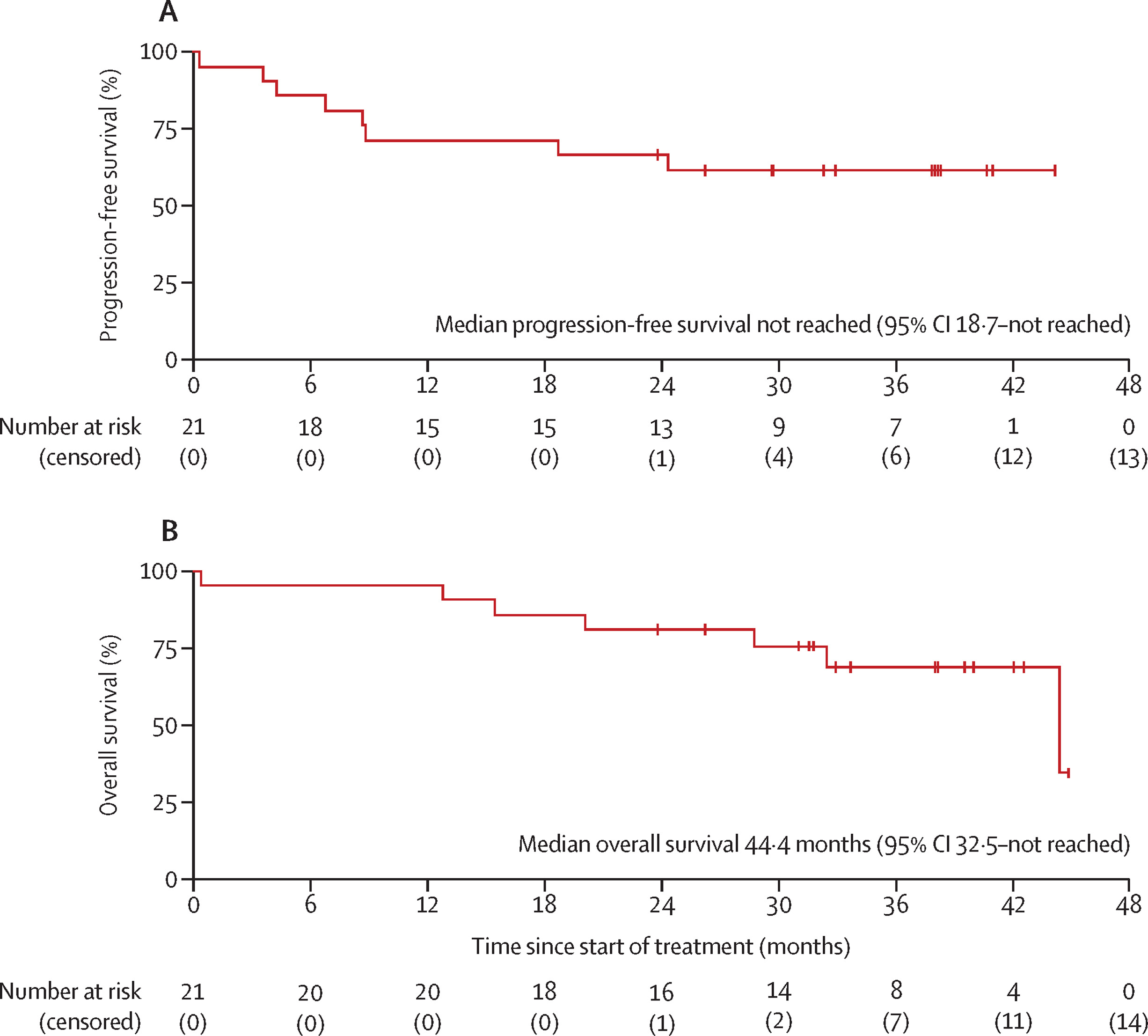
The 2-year progression-free survival (PFS) rate was 67% (90% CI 46–83).
The median PFS was not reached, indicating that many patients remained progression-free at data cutoff.
The median overall survival (OS) was 44.4 months (32.5–NR), with a 2-year OS rate of 81%.
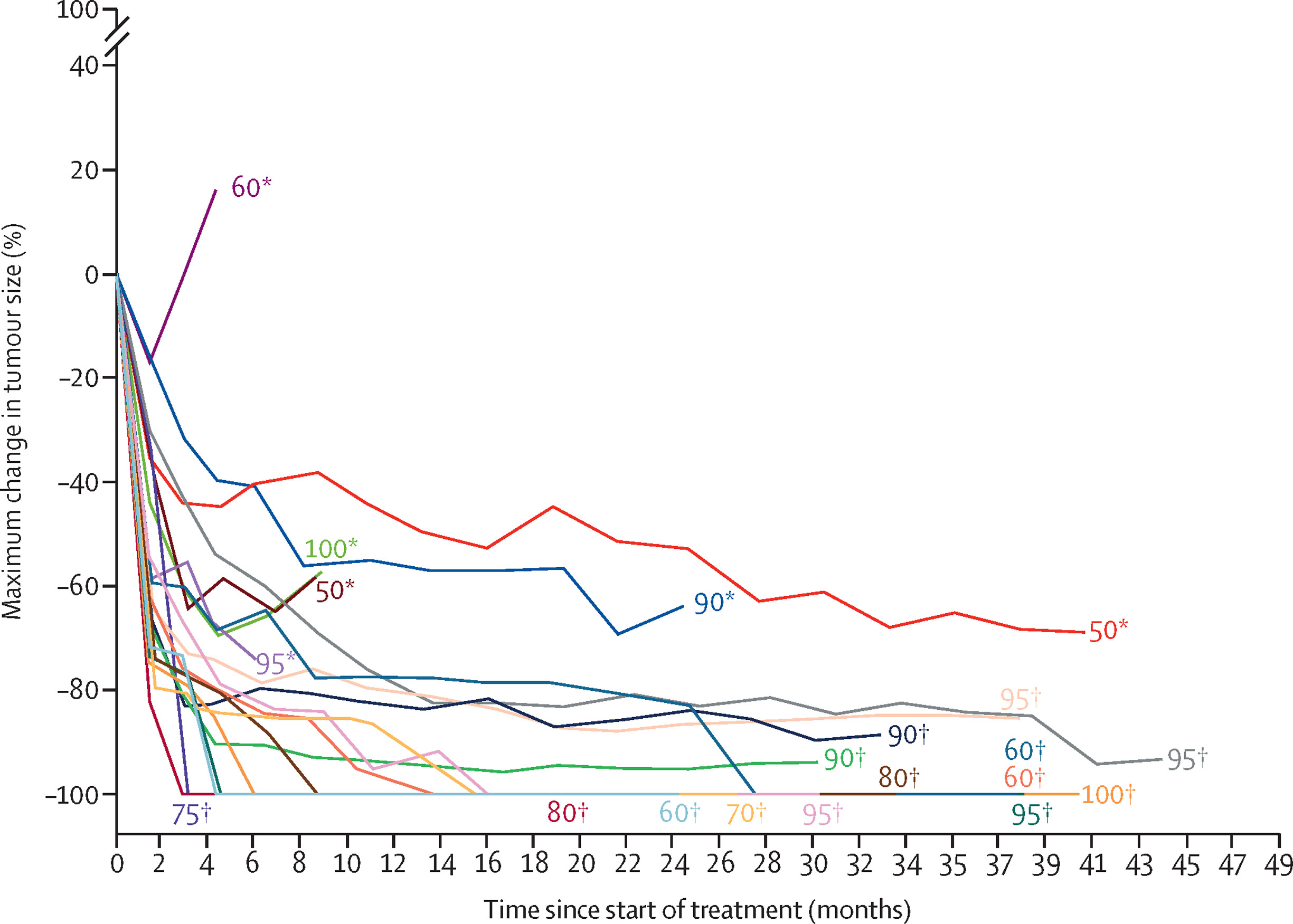
Tumor response was robust. The objective response rate (ORR) was 81%, including 8 complete responses and 9 partial responses. Moreover, two-thirds of patients (67%) achieved deep responses, defined as ≥80% tumor shrinkage. These deep responders had markedly improved outcomes, with a 2-year PFS of 86% compared to 29% in non-deep responders (p = 0.0173), suggesting a strong association between depth of response and long-term disease control.
PD-L1 Expression and Outcomes
Efficacy appeared closely related to the level of PD-L1 expression:
In patients with PD-L1 TPS 80–100%, the 2-year PFS was 75%, OS was 92%, and ORR reached 92%.
In contrast, those with PD-L1 TPS 50–79% had lower rates—PFS 56%, OS 67%, and ORR 67%.
These findings indicate that patients with ultra-high PD-L1 expression (≥80%) experienced the greatest and most durable benefit, reinforcing PD-L1 TPS as a predictive biomarker for selecting candidates who may achieve long-term remission with this radiotherapy-free chemoimmunotherapy approach.
Safety
All 21 patients in the Evolution trial experienced at least one adverse event during treatment, reflecting the expected toxicity profile of combination chemoimmunotherapy. Grade 3 or higher adverse events occurred in 62% of patients, while serious adverse events were reported in 33%. The most frequently observed severe toxicities were neutropenia (38%), leukopenia (19%), and pneumonia (14%), which were consistent with known effects of platinum-based chemotherapy.
Immune-related adverse events (irAEs) were documented in 33% of patients, with grade 3 or higher events in 14%. The most common immune-mediated toxicities included pneumonitis (10%), adrenal insufficiency (5%), and colitis (10%). Importantly, these immune-related events were manageable with corticosteroids and treatment interruption, and no treatment-related deaths were reported.
Overall, 24% of patients discontinued therapy due to adverse events; however, an intriguing observation emerged—three patients who stopped treatment because of immune-related toxicities, such as grade 2–3 pneumonitis or colitis, continued to experience durable disease control lasting beyond two years. This finding supports the concept that a strong immune response, even when accompanied by immune-related toxicity, may correlate with sustained anti-tumor activity and long-term remission in patients treated with immune checkpoint inhibitors.
Interpretation
The Evolution trial provides the first prospective evidence supporting a radiotherapy-free, chemoimmunotherapy approach in unresectable, locally advanced NSCLC with high PD-L1 expression.
Pembrolizumab combined with platinum doublets achieved 2-year PFS and OS rates comparable or superior to historical chemoradiotherapy data with durvalumab consolidation (PACIFIC trial: ~50% 2-year PFS).
The durable responses and survival plateaus observed, particularly among deep responders and PD-L1 ≥80%subgroups, indicate potential curative intent with systemic immunochemotherapy alone.
This approach could represent a less toxic alternative for patients at high risk of radiation-induced morbidity or those ineligible for concurrent chemoradiotherapy. However, the single-arm design and small sample size necessitate randomized validation against the PACIFIC regimen.
Key Takeaway Messages
- Pembrolizumab + chemotherapy without radiotherapy achieved a 2-year PFS rate of 67% and ORR of 81%in unresectable, locally advanced NSCLC with PD-L1 ≥50%.
- Deep responders (≥80% tumor shrinkage) had markedly superior outcomes (2-year PFS 86%).
- PD-L1 ≥80% subgroup showed the highest benefit (2-year OS 92%).
- Safety profile manageable, with no new immune-related toxicities and no treatment-related deaths.
- Findings suggest a potential curative systemic therapy alternative for selected patients; a randomized comparison with chemoradiotherapy–durvalumab is warranted.
You Can Read Full Article Here