At ASCO 2025, Dr. Shilpa Gupta (Cleveland Clinic Taussig Cancer Institute) presented an exploratory analysis from the Phase III EV-302/KEYNOTE-A39 trial focused on patients with locally advanced or metastatic urothelial carcinoma (la/mUC) who achieved a confirmed complete response (cCR). The study reinforces the established role of enfortumab vedotin (EV) plus pembrolizumab (P) as first-line standard of care (SOC) by highlighting superior outcomes in complete responders compared to chemotherapy.
This new subanalysis builds upon the previously reported primary analysis of EV-302, which demonstrated significant progression-free survival (PFS) and overall survival (OS) benefits for EV+P over platinum-based chemotherapy.
What Is EV-302?
EV-302 (KEYNOTE-A39) is a global, randomized Phase III trial evaluating the combination of enfortumab vedotin (1.25 mg/kg on Days 1 and 8) plus pembrolizumab (200 mg on Day 1) every 3 weeks in treatment-naïve patients with la/mUC. The comparator arm received chemotherapy with gemcitabine and either cisplatin or carboplatin.
A total of 886 patients were randomized to receive either EV+P (n=442) or chemotherapy (n=444). While the original trial focused on all patients, this ASCO 2025 analysis zeroes in on the subset of patients who achieved a confirmed complete response—those with the deepest tumor shrinkage.
Key Results Presented at ASCO 2025
At the data cutoff on August 8, 2024, the median follow-up was 29.1 months (95% CI, 28.5–29.9). A total of 886 patients were randomized, with 442 receiving EV+P and 444 receiving chemotherapy. The confirmed overall response rate (ORR), including complete and partial responses, was 67.5% with EV+P versus 44.2% with chemotherapy. The confirmed complete response (cCR) rates were 30.4% and 14.5%, respectively. Baseline characteristics of responders were generally consistent with the intent-to-treat population. Among patients with cCR in the EV+P arm, 28.6% had upper tract disease and 15% had liver metastases.
Survival outcomes were significantly improved in cCR patients receiving EV+P. Median progression-free survival (PFS) was not reached (NR) with EV+P, compared to 26.9 months with chemotherapy (hazard ratio [HR] 0.36; 95% CI, 0.21–0.61). Median overall survival (OS) was not reached in either group but still favored EV+P (HR 0.37; 95% CI, 0.17–0.80).
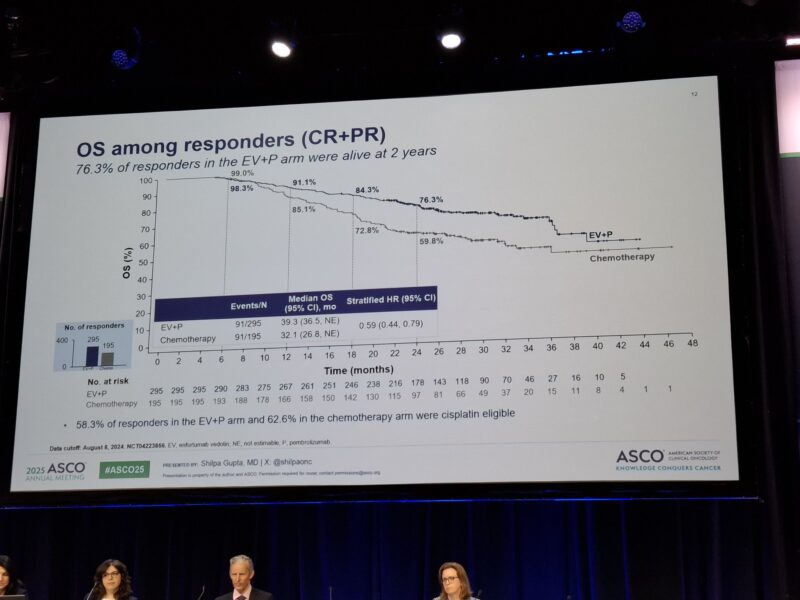
At 24 months, the PFS rate was 78.2% with EV+P versus 53.7% with chemotherapy, while the OS rate was 95.4% versus 85.8%, respectively. The median duration of complete response (DOCR) was not reached with EV+P, compared to 15.2 months with chemotherapy. Additionally, the sustained cCR rate at 24 months was 74.3% with EV+P versus 43.2% with chemotherapy.
These data reflect a durable and deep response across both short- and long-term outcomes, reinforcing the benefit of EV+P as first-line therapy for patients with advanced urothelial carcinoma.
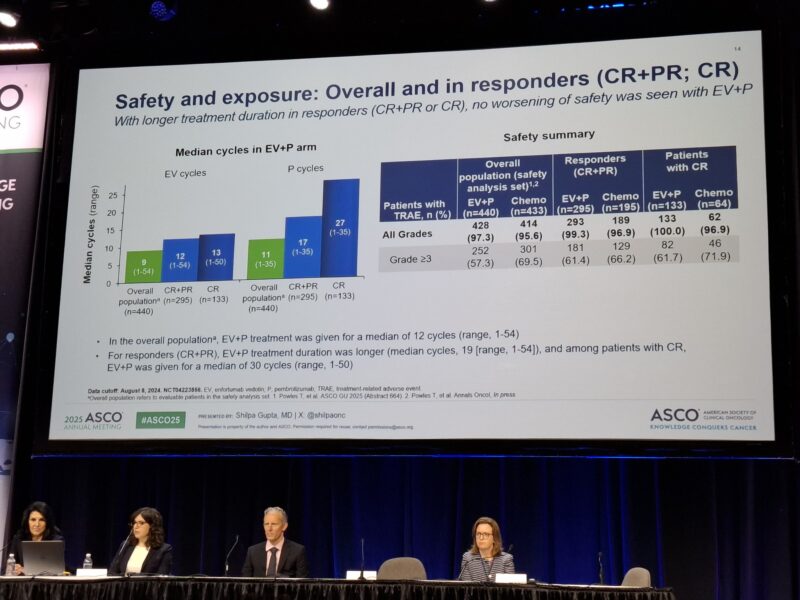
Safety and Treatment Exposure
In patients who achieved a clinical complete response (cCR) and were treated with enfortumab vedotin plus pembrolizumab (EV+P), the safety profile remained consistent with prior findings and was generally manageable. The median treatment duration was 22.0 months, with a median of 13 cycles of enfortumab vedotin administered.
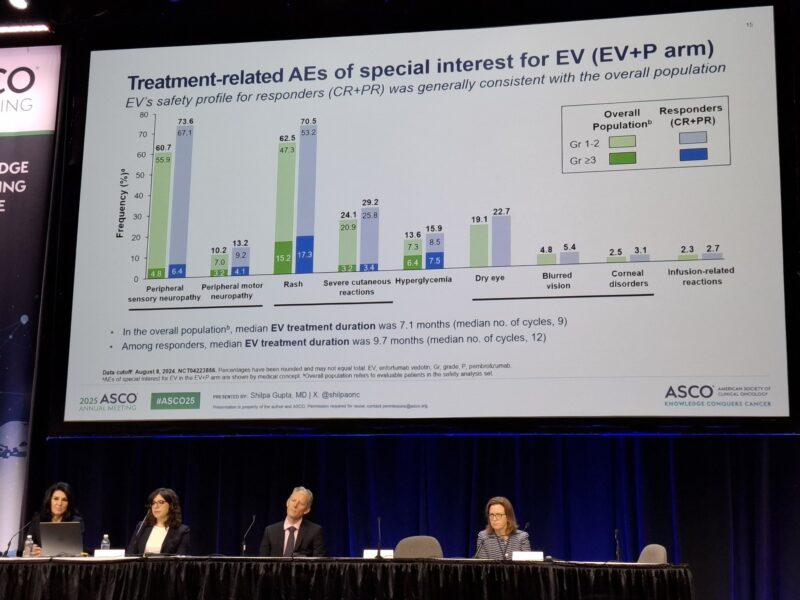
Grade ≥3 treatment-related adverse events (TRAEs) occurred in 61.7% of patients receiving EV+P, compared to 71.9% observed with standard chemotherapy, indicating a more favorable tolerability profile. The most common reasons for dose modifications were fatigue, rash, and peripheral neuropathy—known and expected toxicities with EV. Importantly, no treatment-related deaths were reported in this subgroup.
These results support the manageable safety and long-term treatment feasibility of EV+P in cCR patients with advanced urothelial carcinoma.
Find more information about trial on ASCO official website.
What People Are Saying About EV-302?
Dr. Emre Yekedüz shared exploratory results from the EV-302 trial on his X page, writing:
“EV-302 exploratory: Among pts with confirmed CR in 1L la/mUC
cCR rate:
• EV+P: 30.4%
• Chemo: 14.5%
In cCR pts:
• PFS: NR vs 26.9 mo (HR 0.36)
• OS: NR vs NR (HR 0.37)
• Grade ≥3 TRAEs: 62% (EV+P) vs 72% (chemo)
Durable CRs + manageable safety
Confirms EV+P as SOC in 1L mUC”
Dr. Toni Choueiri shared updated responder data from the EV-302 Phase III trial on his X page, writing:
“ASCO25 | EV‑302 Ph III | la/mUC Responders:
Among pts achieving cCR, EV + P arm showed 74% 2‑yr maintained cCR vs 32% with chemo;
median OS in responders was 33.8 mo vs 16.1 mo (HR 0.51; P<0.001)“
Clinical Takeaways
The combination of enfortumab vedotin and pembrolizumab (EV+P) achieved a confirmed complete response (cCR) in nearly one-third of patients with advanced urothelial carcinoma—more than doubling the cCR rate observed with standard chemotherapy. These responses were not only deep but also durable, with the majority of complete responders remaining progression-free at the 2-year mark.
This pivotal analysis reinforces EV+P as the first-line standard of care for advanced bladder cancer, delivering long-lasting remission even in patients with poor prognostic features such as upper tract involvement and liver metastases. The findings highlight the transformative potential of combining antibody-drug conjugates with immunotherapy, shifting the treatment paradigm from disease control to long-term survival and remission in advanced urothelial carcinoma.
More posts featuring ASCO25.