Esophageal squamous cell carcinoma (ESCC) continues to carry one of the highest cancer burdens in Asia. While neoadjuvant chemoradiotherapy is widely adopted, many patients still undergo surgery alone in real-world practice, creating an unmet need for effective and safe postoperative strategies. The ESO-Shanghai clinical trial program was developed to address exactly this question.
The newly reported ESO-Shanghai 17 study, evaluates whether a small T-shaped radiotherapy field at 50.4 Gy with concurrent paclitaxel–carboplatin can provide adequate loco-regional control while minimizing toxicity. Importantly, the investigators also compared outcomes with the earlier ESO-Shanghai 9 trial, which used a wider extensive field at 45 Gy. This article synthesizes the key findings and clinical implications of both trials.
Methods, Treatment Approach, and Endpoints
ESO-Shanghai 17 was a single-arm, phase II trial conducted at Fudan University Shanghai Cancer Center. Patients were eligible if they were 18–75 years old, ECOG 0–1, had undergone R0 resection within three months, and had pT3–4 or N+ M0 ESCC based on AJCC 8th edition. None received preoperative therapy. Eligibility criteria for ESO-Shanghai 9 were identical, enabling meaningful comparison.
All patients received concurrent chemotherapy with weekly paclitaxel (50 mg/m²) and carboplatin (AUC 2) for up to five cycles. Radiotherapy began on day 1. The distinction between the two trials lay primarily in target volume and dose:
ESO-Shanghai 17
Small T-shaped field, 50.4 Gy in 28 fractions, covering the tumor bed, anastomosis, bilateral supraclavicular nodes, upper mediastinal nodes, and a region 3 cm below the carina.
ESO-Shanghai 9
Extensive field, 45 Gy in 25 fractions, including additional mediastinal and upper abdominal lymphatic regions.
IMRT was used in all patients, with strict organ-at-risk constraints. Follow-up included CT imaging, ultrasound, esophagography, and endoscopy when indicated.
Primary endpoint
- 2-year local control rate in ESO-Shanghai 17, with a predefined success threshold of ≥80%.
Secondary endpoints
- Overall survival (OS)
- Disease-free survival (DFS)
- Locoregional recurrence-free survival (LRFS)
- Distant metastasis-free survival (DMFS)
- Acute and late toxicities (CTCAE v4.0)
Patterns of failure
To reduce confounding when comparing the two trials, the authors performed propensity score matching and multivariable Cox regression analysis, focusing on prognostic factors such as nodal burden and lifestyle exposures.
Results: Efficacy, Failure Patterns, and Survival
The findings of ESO-Shanghai 17 and the joint comparative analysis with ESO-Shanghai 9 were published in Clinical and Translational Radiation Oncology on November 20, 2025, providing updated long-term survival, recurrence patterns, and toxicity outcomes.
ESO-Shanghai 17 enrolled 70 patients between 2020 and 2023, while ESO-Shanghai 9 enrolled 70 patients between 2016 and 2018. Baseline characteristics were broadly comparable, with the majority presenting with stage III ESCC and undergoing two-field lymphadenectomy.
A key technical distinction between the studies was the size of the irradiation field. The median PTV in ESO-Shanghai 17 was nearly half that of ESO-Shanghai 9, translating into significantly lower lung and heart doses while maintaining spinal cord constraints. Radiotherapy completion rates exceeded 90% in both cohorts.
ESO-Shanghai 17 successfully met its primary objective. The 2-year local control rate reached 83.8%, surpassing the predefined 80% threshold. Survival outcomes were highly similar between the two strategies, with parallel OS, DFS, LRFS, and DMFS curves. After propensity score matching, the treatment regimen (T-shaped vs extensive field) did not independently affect survival, whereas the number of positive lymph nodes remained the strongest prognostic factor across all survival endpoints.
To highlight key outcomes
- Dose & anatomy: ESO-Shanghai 17 achieved markedly lower lung V5/V20 and mean heart dose due to its smaller target field.
- Local control: 2-year local control was 83.8% in ESO-Shanghai 17 vs 87.9% in ESO-Shanghai 9 (not statistically different). Both values fall within overlapping confidence intervals, consistent with the non-significant difference reported.
- Survival: OS rates at 2 years were 84.0% (ESO-Shanghai 17) vs 73.9% (ESO-Shanghai 9), with no statistically significant differences in OS, DFS, LRFS, or DMFS.
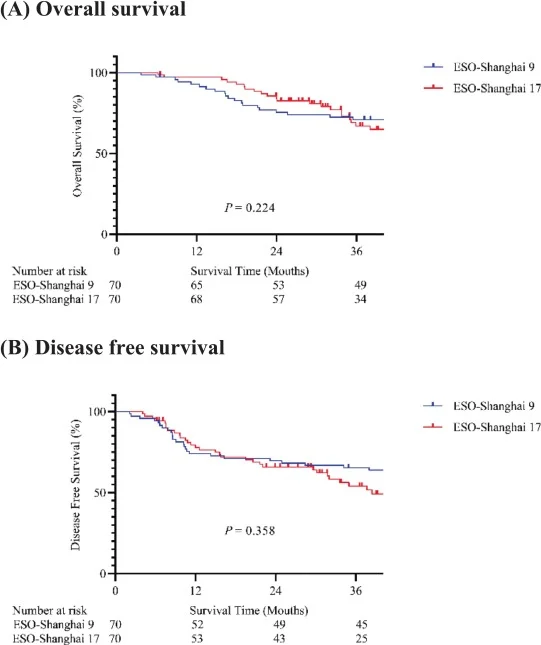
- Failures: Locoregional recurrence as the first site of failure occurred in 24.3% (ESO-Shanghai 17) vs 12.9% (ESO-Shanghai 9), while distant metastasis rates were nearly identical at ~22%. Although the absolute rate of locoregional failure was numerically higher with the small T-shaped field, this difference did not reach statistical significance, and survival endpoints remained comparable, highlighting the need for confirmation in larger randomized cohorts.
- Toxicity: Hematologic toxicities—especially grade ≥3 leukopenia and neutropenia—were significantly less frequent in ESO-Shanghai 17.
- Safety: Two pneumonitis-related deaths occurred in ESO-Shanghai 9, while none occurred in ESO-Shanghai 17, aligning with significantly lower lung dose parameters.
Taken together, the results support that the smaller T-shaped radiotherapy field provides comparable oncologic efficacy while substantially improving safety, especially regarding pulmonary risk.
Conclusion
The ESO-Shanghai 17 study demonstrates that postoperative chemoradiotherapy using a 50.4 Gy small T-shaped field offers local control and survival outcomes comparable to the broader-field ESO-Shanghai 9 regimen, while reducing hematologic toxicity and avoiding pneumonitis-related mortality.
For patients undergoing surgery alone for pT3–4 or N+ M0 ESCC, this focused postoperative approach appears effective and may be safer—particularly for individuals with higher pulmonary or cardiac risk.
While the comparison is limited by the non-randomized, sequential nature of the trials, the findings reinforce that refining radiation volume—not only radiation dose—can meaningfully improve tolerability without compromising oncologic outcomes. At the same time, the numerically higher local failure rate with the small T-shaped field, despite similar LRFS, suggests that careful patient selection and longer-term follow-up remain essential. Larger randomized trials incorporating modern systemic therapy are warranted to define the optimal postoperative strategy for ESCC in the contemporary era.