Physical activity has long been promoted as part of healthy survivorship after colon cancer, yet until recently, recommendations were largely based on observational and preclinical evidence. However, the absence of randomized evidence demonstrating a causal relationship between exercise and oncologic outcomes limited its formal integration into clinical practice guidelines.
In January 2026, ESMO Open published an Express Update to the ESMO Clinical Practice Guidelines for localised colon cancer, specifically addressing the role of structured physical exercise in patients with resected disease. This update incorporates new high-level evidence and provides formal recommendations for clinical practice.
Background and Rationale
Colon cancer is the third most common cause of cancer-related death globally. The reported increase in incidence and shift toward early-age carcinogenesis are partly attributed to lifestyle-related factors, including obesity, reduced physical activity, alcohol consumption, high red meat intake, smoking, and imbalances in the microbiota.
Preclinical and observational studies have correlated higher levels of recreational physical activity after anticancer therapy with a lower risk of malignant recurrence and death, suggesting potential metabolic, inflammatory, and immune-modulating mechanisms. However, until recently, high-level evidence defining the clinical benefit, intensity, and structure of exercise programmes in colon cancer survivors was lacking.
Evidence Informing the Update
The guideline update is primarily based on the phase III Canadian Cancer Trials Group CHALLENGE trial, which randomized 962 patients with resected stage III or high-risk stage II colon cancer who had completed standard adjuvant chemotherapy.
Eligible patients had completed adjuvant chemotherapy within the previous 2–6 months, had an ECOG performance status of 0–1, and were able to complete at least two stages of a submaximal treadmill test or the 6-minute walk test.
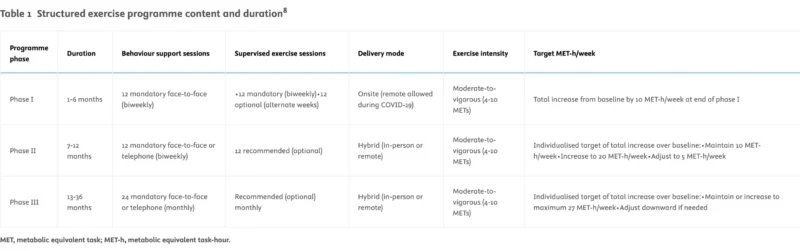
The exercise intervention included an exercise guidebook and long-term support from certified physical activity consultants. The support programme included 17 evidence-based behavioural change techniques delivered over three phases, using a combination of mandatory and optional sessions, in-person and remotely. The programme aimed to increase recreational aerobic activity by at least 10 MET-hours per week, equivalent to approximately 150 minutes of moderate-intensity aerobic exercise, and to maintain or further increase this level over time.
Study endpoints
- Primary endpoint: Disease-free survival (DFS), defined as time to recurrence, new primary cancer, or death from any cause
- Secondary endpoints: Overall survival, quality of life, physical fitness, safety, programme adherence, and economic evaluations
Results
At a median follow-up of 7.9 years, disease-free survival (DFS) was significantly improved in the exercise group compared with the health education group:
- DFS: HR 0.72 (95% CI 0.55–0.94), P = 0.02
- 5-year DFS: 80.3% vs 73.9%
Overall survival was also improved:
- OS: HR 0.63 (95% CI 0.43–0.94)
The exercise intervention achieved and sustained the targeted increase in moderate-to-vigorous physical activity throughout the 3-year programme and was associated with meaningful improvements in cardiorespiratory fitness and physical functioning.
Safety
Adherence declined over time, particularly during later programme phases:
- Behavioural support adherence decreased from 83% to 63%
- Supervised exercise adherence declined from 79% (months 1–6) to 44% (months 13–36)
Musculoskeletal adverse events were more frequent in the exercise group (18.5% vs 11.5%), including joint, back, limb, and muscle pain. Grade ≥3 musculoskeletal events were uncommon (0.7% vs 0.2%), and overall grade ≥3 adverse events occurred in 15.4% of exercise participants compared with 9.1% in the health education group, largely driven by musculoskeletal events.
Guideline recommendations
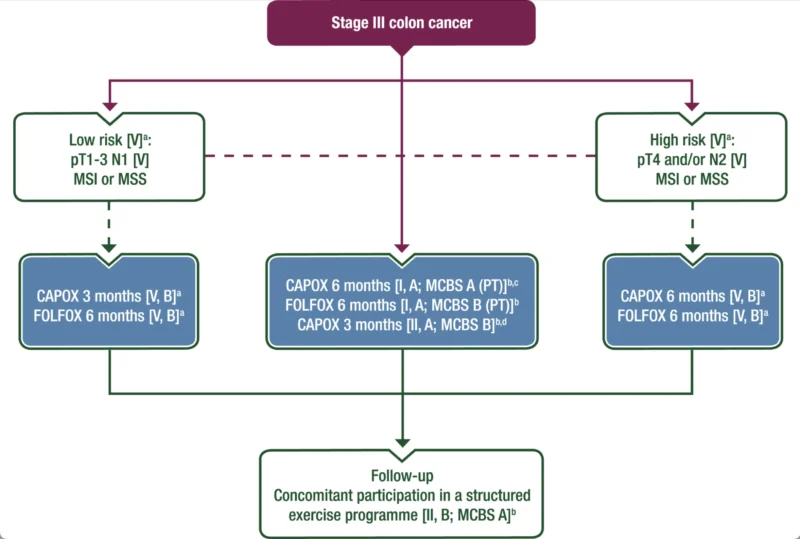
Based on these findings, the ESMO Guidelines Committee recommends that patients with resected stage III or high-risk stage II colon cancer be informed about the available evidence supporting survival benefits from participation in a structured exercise programme. Health care professionals should assess eligibility, discuss feasibility, and consider patient preferences, comorbidities, and potential barriers, including infrastructure and financial toxicity.
After shared decision-making, participation in a structured exercise programme may be recommended. The clinical benefit of this intervention has been assigned an ESMO-Magnitude of Clinical Benefit Scale (MCBS) v2.0 score of A. Health system investment can be recommended to support the development of behavioural support and exercise infrastructures.
More broadly, patients with resected localised colon cancer should be encouraged to maintain a healthy lifestyle, including regular physical activity (approximately 10 MET-hours per week), smoking cessation, avoidance of excessive alcohol intake, and adoption of a healthy diet rich in vegetables, fruit and berries, adapted to gastrointestinal function.
Conclusion
This Express Update represents a substantive development in the management of localised colon cancer. Structured physical exercise is supported by randomized evidence demonstrating improvements in disease-free and overall survival and is formally incorporated into ESMO clinical practice guidelines. The update reinforces the role of structured survivorship interventions as an integral component of post-treatment care for selected patients with resected colon cancer.
The full guideline update is available in ESMO Open.



