Title: Enfortumab Vedotin or Chemotherapy in the Sequential Treatment of Advanced Urothelial Carcinoma: The ARON-2 Retrospective Experience
The ARON-2 study evaluates the real-world effectiveness of enfortumab vedotin (EV) in the third-line treatment of advanced urothelial carcinoma (aUC), offering insights into its efficacy, progression-free survival (PFS), and overall survival (OS) compared to chemotherapy. This large, multicenter retrospective analysis highlights the significant benefits of EV over traditional chemotherapy, emphasizing its potential to improve patient outcomes in third-line settings after platinum-based chemotherapy and pembrolizumab. The findings support EV’s integration into standard aUC treatment protocols, reinforcing its role in enhancing survival and response rates.
Authors
Mimma Rizzo, Franco Morelli, Yüksel Ürün, Sebastiano Buti, Se Hoon Park, Maria T. Bourlon, Enrique Grande, Francesco Massari, Johannes Landmesser, Alexandr Poprach, Hideki Takeshita, Giandomenico Roviello, Zin W. Myint, Lazar Popovic, Andrey Soares, Halima Abahssain, Patrizia Giannatempo, Javier Molina-Cerrillo, Lorena Incorvaia, Samer Salah, Annalisa Zeppellini, Fernando Sabino Marques Monteiro, Camillo Porta, Shilpa Gupta, Matteo Santoni
Published in Cancer Medicine, Feb 2025
Background
Advanced urothelial carcinoma (aUC) remains a challenging disease with limited treatment options beyond first- and second-line therapy. Enfortumab vedotin (EV), an antibody-drug conjugate (ADC), has shown promising efficacy in patients previously treated with platinum-based chemotherapy and immune checkpoint inhibitors. The ARON-2 study, a large, international, multicenter, retrospective analysis, evaluates real-world third-line treatment patterns and outcomes, comparing EV with chemotherapy.
Methods
In ARON-2 study a total of 1,039 patients with aUC who had received platinum-based chemotherapy followed by pembrolizumab were analyzed. Of these, 247 patients (23.8%) underwent third-line treatment. Data were collected from multiple centers worldwide, assessing overall response rate (ORR), progression-free survival (PFS), and overall survival (OS). Subgroup analyses explored treatment outcomes based on prior therapy, histology, and patient performance status (ECOG PS).
Study Design
This retrospective observational study compared third-line EV and chemotherapy in aUC patients. Key endpoints included ORR, median PFS, and median OS. The study also evaluated the impact of prior treatments and patient characteristics on survival outcomes. Statistical comparisons were made to assess EV’s efficacy relative to chemotherapy.
Results
Among patients who progressed on platinum drug and immunotherapy, third line Enfortumab vedotin (EV) demonstrated significantly superior efficacy compared to chemotherapy.
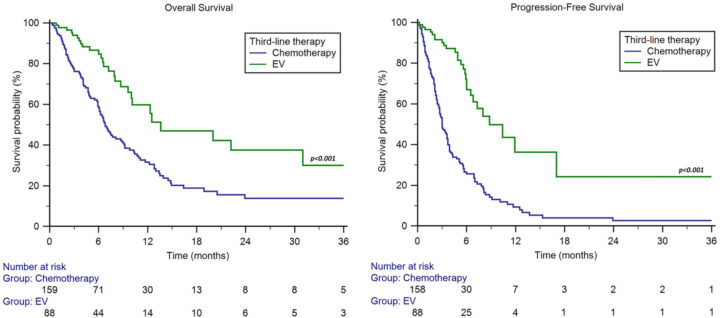
- Among 159 patients receiving third-line chemotherapy post-pembrolizumab, the ORR was 23%, and median PFS was 3.0 months (95% CI: 2.6–3.7).
- Patients treated with EV had superior outcomes:
- Median OS: 13.6 months vs. 6.8 months for chemotherapy.
- 1-year OS rate: 60% vs. 32%.
- Median PFS: 8.8 months for EV vs. 3.0 months for chemotherapy.
- 1-year PFS rate: 36% vs. 9%.
- ORR: 56% with EV vs. 23% with chemotherapy.
- Duration of response (DoR): 17 months with EV vs. 8 months with chemotherapy.
- Subgroup analysis showed consistent OS benefits across tumor histology, metastatic burden, and prior platinum agent used.
- Patients receiving EV had favorable performance status (ECOG 0-1 in 82%).
- The real-world EV outcomes were comparable to those from the EV-301 trial and superior to historical chemotherapy data.
Key Findings
The ARON-2 study revealed that third-line treatment with enfortumab vedotin (EV) significantly outperformed chemotherapy in advanced urothelial carcinoma (aUC).
- Third-line treatment utilization was higher in this global study (23.8%) than in US-based data (11.8%), possibly due to treatment access and patient selection.
- EV significantly improved survival outcomes compared to chemotherapy, with a nearly twofold increase in median OS and ORR.
- EV efficacy was consistent across subgroups, indicating broad clinical benefit.
- Chemotherapy following pembrolizumab showed modest efficacy, aligning with previous retrospective studies.
- Indirect immune modulation by cytotoxic agents may enhance CD8+ T-cell activity and contribute to the observed benefits.
Key Takeaway Messages
- Enfortumab vedotin is an effective third-line option in aUC, demonstrating superior survival benefits over chemotherapy.
- Its efficacy appears independent of prior platinum therapy or response to pembrolizumab.
- The real-world data reinforce EV’s clinical utility and support its integration into standard treatment algorithms.
- Further research is needed to optimize sequential therapy strategies and identify predictive biomarkers.
Conclusion
This large, international real-world analysis supports the effectiveness of enfortumab vedotin in third-line treatment for aUC. Compared to chemotherapy, EV significantly improves survival outcomes, with higher response rates and longer PFS. While retrospective study limitations exist, including potential selection bias and lack of genomic data, the findings offer valuable insights into treatment sequencing and reinforce EV’s role as a preferred third-line option. Future prospective studies are needed to refine patient selection and optimize combination strategies.
You Can Read the Full Article Here
Summary by Sona Karamyan


